Acid rain was first discovered in 1948 in Sweden. Acid rain is a phenomenon where rainwater has a low pH (below 5.6) and contains nitrogen and sulfur. The root cause of acid rain stems from human consumption of natural resources such as coal and oil for living and production processes.
Causes of acid rain.
Acid rain can also originate from volcanic eruptions, wildfires, or lightning, where sulfur dioxide (SO2) and nitrogen dioxide (NO2) combine with water vapor in the atmosphere to form acids in two forms: dry as gas and wet as acid rain, snow, or fog.
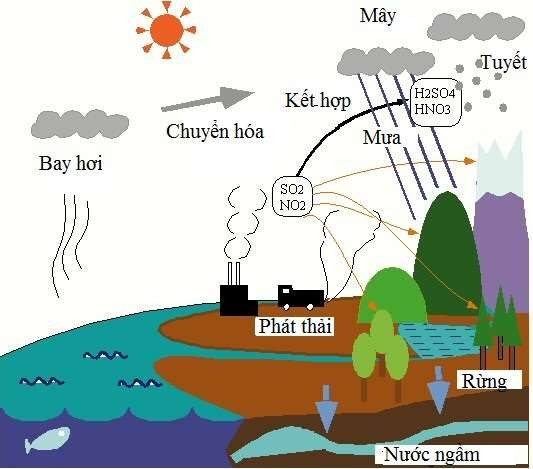
The process of acid rain formation.
Acid rain with a pH lower than 4.7 converts organic lead in the soil into inorganic lead, inhibiting cell division in plant roots. Consequently, spores of bacteria, fungi, viruses, and other pathogens can invade the roots and potentially kill the plants. If the pH value drops below 3.5, it directly harms the plant canopy, causing wilting and death. Therefore, this type of acid rain is often referred to as “the grim reaper in the sky.”
Effects of Acid Rain
The consumption of coal and oil releases large amounts of harmful gases, namely sulfur dioxide (SO2) and nitrogen dioxide (NO2). Once released into the environment, these gases dissolve with water vapor in the air, forming sulfuric acid (H2SO4) and nitric acid (HNO3). When it rains, acid particles mix with the water, lowering the pH of the rainwater. It can dissolve some metal dust and metal oxides suspended in the air, such as lead oxide, becoming toxic to plants, livestock, and humans.

Acid rain adversely affects the soil by leaching into the ground, increasing soil acidity.
- Acid rain negatively impacts water bodies (ponds, lakes). The runoff from acid rain into lakes and ponds quickly reduces their pH, weakening or completely killing the organisms within.
- Acid rain can cause significant harm to forest vegetation. The longer tree leaves are exposed to acid rain, the more severe the damage.
- Acid rain adversely affects the soil as rainwater leaches into the ground, increasing soil acidity and dissolving essential soil elements for plants, such as calcium (Ca) and magnesium (Mg), leading to soil degradation and poor plant growth. Leaves exposed to acid rain may develop “burn” spots, and buds may die off, reducing the plant’s photosynthesis capacity and yield.
- Acid rain also damages metal materials such as iron, copper, and zinc, reducing the lifespan of construction works.
- The direct health effects of pollution from acidic gases on humans include respiratory diseases such as asthma, whooping cough, and other symptoms like headaches, eye pain, and sore throat. Indirect effects arise from the bioaccumulation of metals in the human body from food sources contaminated by these metals due to acid rain.
- Sulphate and nitrate particles formed in the atmosphere can limit visibility. Acid fog affects the transmission of sunlight. In the Arctic, it has impacted the growth of lichens, thereby affecting populations of reindeer and caribou—animals that feed on lichens.
How to Reduce Acid Rain?
Despite clear evidence related to air pollution, companies have denied responsibility and questioned research findings. In the United States, companies have lobbied against pollution regulations and persuaded politicians that such policies would increase energy costs and threaten jobs. These obstacles led the government to delay changes and pause further research on the issue.
However, after a decade, Congress finally took action. Since most sulfur dioxide emissions come from power plants, the government set limits on emissions for each plant annually. This forced all companies to reduce emissions in the long term.
Some plants have added desulfurization filters to their smokestacks or switched to low-sulfur coal and natural gas. These advancements have allowed the electricity sector to grow while controlling pollution.
By 1985, Canada and the European Union had implemented their own solutions, and international agreements began to circulate to reduce air pollution worldwide. Some countries, like Russia, India, and China, still rely heavily on high-sulfur coal. However, recognizing acid rain as a significant threat has led to the implementation of many environmental protection policies to mitigate this danger. In conclusion, acid rain is a serious but manageable threat, depending on whether nations are decisive in addressing it.