A new study has discovered that dissolving a simple sugar into the electrolyte of flow batteries can increase their maximum power output by 60%.
Researchers from the Pacific Northwest National Laboratory (PNNL) found that dissolving a simple sugar into the electrolyte of flow batteries significantly boosts their maximum power output by 60%. Furthermore, after being continuously used for a year, the batteries show almost no loss in capacity. The study was published in the journal Joule.
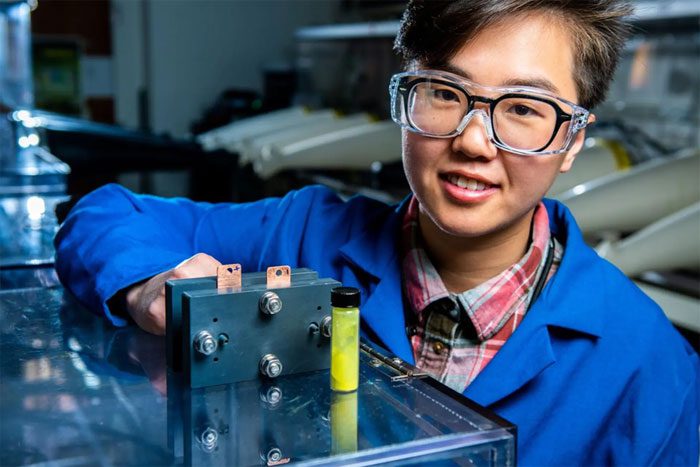
Scientists testing the dissolution of a sugar called β-cyclodextrin into the anolyte of the cell.
Flow batteries are typically best suited for large-scale, long-duration energy storage applications. This has led to increased interest in this type of battery in recent years. Generally, flow batteries consist of two liquids, anolyte and catholyte, stored separately.
These liquids are then pumped through either side of an ion-selective membrane, generating electricity when energy is needed. This setup offers certain advantages.
For instance, users can “refuel” their system by swapping depleted electrolytes for charged ones. Alternatively, they can recharge the system using grid power to reverse the discharge cycle.
The researchers from the Pacific Northwest National Laboratory were the first to experiment with dissolving a sugar known as β-cyclodextrin into the anolyte of the cell. The team hopes that this sugar will help address a completely different challenge.
Ruozhu Feng, the lead author of the study, stated: “We are looking for a simple way to dissolve more fluorenol in the water-based electrolyte. β-cyclodextrin has modestly helped achieve that, but its real benefit is this remarkable catalytic ability.”
In collaboration with researchers from Yale University, the team uncovered the mechanism behind this enhancement. The sugar captures positively charged protons, balancing the movement of negatively charged electrons moving to and fro across the cell membrane. After some adjustments, the reaction rate can increase, thus raising the effective energy level of the battery by up to 60%.
This type of sugar is completely dissolved in the electrolyte, contrasting with the Influit flow battery technology developed from research at Illinois Tech, which utilizes small, solid nanoparticles of active metal oxide battery material suspended in a viscous liquid.
Wei Wang, a battery researcher at PNNL and the principal investigator of the study, noted: “This is a completely new approach to developing electrolytes for flow batteries. We have demonstrated that a completely different type of catalyst can be used to accelerate the energy conversion process. Moreover, since it is dissolved in the liquid electrolyte, it eliminates the potential for solids to dislodge and contaminate the system.”