Antoine-Laurent de Lavoisier, a distinguished French chemist of the 18th century, is often referred to as the “father of modern chemistry” due to his meticulous experiments and groundbreaking discoveries that transformed the way humanity understands chemical reactions.
His work laid the foundation for key principles, including the law of conservation of mass and the systematic nomenclature of chemical substances.
Antoine-Laurent de Lavoisier is known as the “father of modern chemistry.”
Born in 1743 in Paris, France, Antoine Lavoisier grew up in a wealthy family. Recognizing his son’s passion for science, Lavoisier’s father, a prominent lawyer working in the French Parliament, provided him with a solid education in mathematics, astronomy, and botany.
Lavoisier studied law, graduated from the University of Paris, and was licensed to practice law in 1764, but his true passion lay in the natural sciences.
He pursued scientific research independently and became a member of the French Academy of Sciences at the young age of 25, marking the beginning of his illustrious scientific career, according to Chemistryviews.
Challenging Conventional Wisdom: The Invention of the Law of Conservation of Mass
One of Lavoisier’s most significant contributions was refuting the prevalent Phlogiston Theory of the 17th century. At that time, it was believed that a substance called phlogiston was released during combustion, explaining why burning materials and metals gained weight when heated.
Lavoisier, with his meticulous approach, conducted a series of experiments that challenged this theory. He demonstrated that combustion is not a release but rather a process where substances combine with oxygen from the air.
Lavoisier’s discoveries led to the recognition of oxygen as a crucial element in combustion, revolutionizing the understanding of chemical reactions.
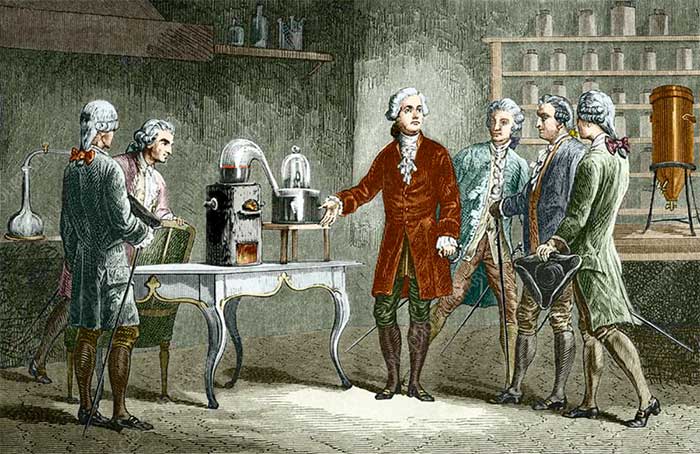
He discovered many revolutionary new theories in chemistry.
Lavoisier’s experiments on combustion and chemical reactions also led him to formulate the law of conservation of mass. Through precise measurements, he showed that the total mass of reactants in a chemical reaction equals the total mass of the products formed.
This groundbreaking principle provided the foundation for understanding the fundamental concept of matter conservation, establishing the basis of modern chemistry. The Lavoisier Law profoundly impacted the development of chemistry, emphasizing the importance of accurate measurements and quantitative analysis in scientific research.
Additionally, Lavoisier recognized the necessity of systematically naming chemical substances. Together with chemist Louis-Bernard Guyton de Morveau, he developed a comprehensive nomenclature system for classifying and naming chemical elements and compounds based on their properties.
This revolutionary approach brought order and consistency to the chaotic naming conventions that existed at that time. Lavoisier’s system laid the groundwork for modern chemical nomenclature, and many names he proposed, such as Oxygen and Hydrogen, are still in use today.
Antoine Lavoisier’s contributions to the field of chemistry are groundbreaking and far-reaching. His emphasis on precise measurements, the refutation of the Phlogiston Theory, and the establishment of the law of conservation of mass revolutionized the study of chemical reactions.
Lavoisier’s systematic approach to naming chemical substances brought order to a previously undefined field.
Execution Shocks the Scientific Community
Sadly, Antoine Lavoisier’s life ended too soon and unjustly amid the chaos of the French Revolution. Despite his immense contributions to science, Lavoisier’s earlier work as a tax collector led to his arrest and eventual execution.

Lavoisier’s life came to a brief end simply because he was a tax official.
During the French Revolution, the radicals sought to eradicate all remnants of the old regime and decided to execute tax officials, including Antoine-Laurent Lavoisier. His contributions to the advancement of chemistry and his reputation as a scientist were completely overlooked.
Lavoisier’s pleas to continue his scientific work and his offers of collaboration for the benefit of science were ignored. At the age of 50, Antoine Lavoisier was led to the guillotine along with 27 other individuals. His execution sent shockwaves through the scientific community, as one of the greatest minds of the time was lost.
Despite his unjust fate, Lavoisier’s scientific legacy endures. His ideas and discoveries continue to shape the field of modern chemistry and inspire future generations of scientists.