At the beginning of the 20th century, two major pandemics occurred: smallpox in 1913 and the Spanish flu in 1919. The Spanish flu reached Australia as soldiers returned from Europe after World War I, infecting the local population. At the peak of the flu outbreak, up to 36% of the population was infected, resulting in a mortality rate of 1.4%. In Sydney, the New South Wales state government ordered the closure of public theaters, required citizens to wear masks on public transportation and in workplaces.
Schools and pubs were closed, and even church services and horse racing events were canceled. Following the Spanish flu, there were two other significant flu pandemics that spread worldwide: the flu of 1957 and 1968, but their consequences were not as devastating as the 1918 outbreak. This serves as a chilling lesson about pandemics, as health professionals and organizations in many countries today are concerned about avian influenza spreading to humans and potentially becoming a rapidly transmissible and lethal infection.
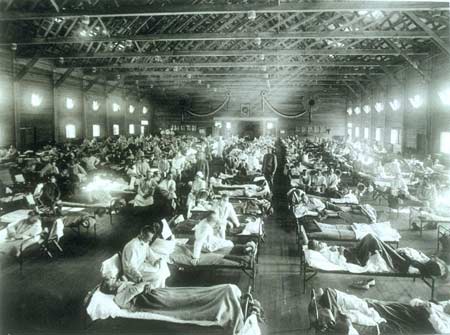
Devastating Spanish flu epidemic of 1918 (Image: fluwikie)
Influenza Viruses
Influenza viruses are categorized into three types: A, B, and C. Type A viruses, which affect chickens, ducks, pigs, whales, and humans, are the most dangerous. Type A viruses are further divided into groups based on two types of proteins: hemagglutinin (HA) and neuraminidase (NA) on the surface of the virus. Scientists have identified 16 types of HA (H1-H16) and 9 types of NA (N1-N6). This means there are a total of 144 combinations of influenza A viruses, but only 3 combinations (H1N1, H1N2, H3N2) are currently known to be transmissible between humans.
Avian influenza belongs to the H5N1 virus type, which scientists fear may join the list of viruses transmissible among humans. This strain first appeared in 1997 in Hong Kong, killing thousands of chickens and 6 humans. After authorities destroyed over 1.5 million chickens, the outbreak ceased, but the H5N1 avian influenza virus remained in circulation. In February 2004, a man died from avian influenza in Hong Kong, followed by an outbreak in January 2004 that killed chickens in South Korea, Vietnam, Japan, and Thailand. By the end of February 2004, a total of 28 people had been infected with H5N1, of which only 7 survived. Avian influenza quickly spread to Cambodia, Laos, and Indonesia.
Through migratory birds carrying the virus from one location to another, the spread of avian influenza accelerated. By the end of 2005, it had reached Turkey, and recently in January and February 2006, it spread to Nigeria (Africa), Bulgaria, and France.
Could H5N1 Avian Influenza Become a Deadly Pandemic Like the 1918 Spanish Flu?
The Spanish flu virus is a variant of the H1N1 type. It is now understood that the Spanish flu virus originated from birds and jumped to humans, according to recent research. The genetic makeup of the Spanish flu virus has been sequenced, revealing that the current avian influenza virus circulating in Asia has genetic alterations very similar to the Spanish flu virus that caused rapid and lethal transmission. Only a few specific proteins in the virus’s protein structure can turn it into a deadly strain. Unlike common influenza viruses, the Spanish flu virus infects cells deep in the lungs and surrounding alveolar cells, which are typically not targeted by regular influenza viruses.
 |
|
H1N1 Virus (Image: spiegel) |
In laboratory settings, unlike common viruses, both the Spanish flu and the current human-adapted avian viruses kill mice upon infection. However, the H5N1 avian influenza virus has not yet been transmitted from human to human.
In 1995, scientist Jeffery Taubenberger from the Molecular Pathology Department at the Armed Forces Institute of Pathology (USA) set out to rediscover the Spanish flu virus for research, hoping it would reveal the genetic changes that allow for human-to-human transmission, thus helping humanity develop preventive measures before such pandemics spread widely. This scientific research is crucial for identifying dangerous viruses and methods to neutralize them. In 1918, the virus had not yet been discovered by science. How could scientists today rediscover the Spanish flu virus and reconstruct its genetic structure?
Taubenberger recalls that his institute had a repository of cellular samples from autopsies established during President Abraham Lincoln’s administration, which mandated that whenever a military physician diagnosed a disease and collected tissue samples, a portion of that tissue must also be sent to and stored at the Armed Forces Institute of Pathology. Taubenberger wanted to see if he could find lung cells from soldiers who died during the 1918 flu pandemic and extract the virus for study. He found tissue samples from two soldiers, their lung samples preserved in formalin within wax blocks. These lung tissues had been stored and untouched for nearly 80 years, yet the virus had deteriorated to only a few viral molecules. Fortunately, he managed to obtain a third sample from a woman who died in Alaska when the flu reached her village, killing 72 people and leaving only 5 survivors.
 |
|
Scientist Jeffery Taubenberger researching the virus behind the 1918 Spanish flu (Image: gazette) |
All victims were buried in a mass grave beneath permanently frozen ground (permafrost). John Holtin, a retired pathologist, learned about Taubenberger’s research and personally financed his trip from San Francisco to the burial site in Alaska, where he obtained lung cell samples that remained frozen, sending them to Taubenberger. For nearly a decade, Taubenberger’s team extracted and reconstructed the genes of the Spanish flu virus. They published the genetic sequence of the 8 viral genes in scientific journals Nature and Science.
In August 2005, Tumpey and colleagues at the Center for Disease Control used the genetic information published by Taubenberger to reconstruct the 1918 virus. They wondered what would happen if laboratory mice and human lung tissues infected with the reconstructed 1918 virus were tested. The scientists exercised extreme caution, using specialized labs to protect researchers and prevent the spread of the reconstructed 1918 virus. They also questioned whether the 1918 virus would remain lethal if they replaced some genes in the 8 reconstructed genes with genes from currently circulating influenza viruses. Scientists began to unveil the secrets of the 1918 virus.
In gene exchange experiments, they replaced the hemagglutinin gene from the 1918 virus with the hemagglutinin gene from the most recent human influenza virus. The surprising result was that the reconstructed 1918 virus no longer proliferated in the lungs of mice and failed to kill them. Additionally, it could not attach to lung tissue cells in laboratory settings. Most importantly, the protein on the hemagglutinin gene of the lethal 1918 virus differed from that of the avian influenza virus by only two amino acids. This indicates the potential danger and unpredictable consequences of the current avian influenza virus if its hemagglutinin gene mutates or exchanges genes with other human influenza viruses to become similar to the hemagglutinin gene of the 1918 virus.
Zoonotic Diseases
 |
| Protozoa Falciparum malaria (Image: cmpharm) |
Jared Diamond shows that when humans domesticated animals into livestock, this close contact led to many infectious diseases caused by bacteria and viruses adapted from animals to humans. Smallpox and tuberculosis originated from bacteria in cattle adapting to human transmission. AIDS began from a mutated virus jumping from monkeys to humans, while malaria is caused by the protozoan Falciparum malaria, which originated from similar protozoa in birds.
Surveys of zoonotic diseases indicate four evolutionary stages of viruses and bacteria that cause diseases in humans. The first stage includes diseases that we occasionally contract directly from livestock, such as cat scratch fever, leptospirosis from pigs, dogs, and cats, and psittacosis from chickens and parrots. These bacteria are still in the early stages of evolution towards becoming transmissible between humans and have not yet directly transmitted from one person to another. The second stage shows that bacteria evolve to the point where they can be directly transmitted between humans and cause infectious outbreaks.
However, some of these infectious diseases have disappeared due to reasons such as being controlled by modern medicine or ceasing when the group of infected individuals became immune or died off, no longer transmitting the disease. For example, in 1959 in East Africa, a fever known as Onchocerciasis fever emerged, infecting several million people. This disease may have originated from a virus in monkeys and transmitted to humans via mosquitoes. Because patients recovered quickly and became immune, this new disease vanished rapidly.
 |
| Health workers during the Lassa fever outbreak in Nigeria in 1969 (Photo: terasz) |
The third stage in the evolution of infectious diseases from microorganisms in animals is when they are present in humans and have not disappeared; they can re-emerge and spread rapidly without warning. For instance, the lethal Lassa fever, likely caused by a virus from rats, spread so quickly in Nigeria in 1969 that hospitals had to close after just one case of hospitalization. The final evolutionary stage is infectious diseases that have occurred in humans multiple times and remain present; they have firmly established themselves in human society after undergoing several evolutionary stages from animals to humans and spontaneously arising from person to person.
Dealing with and Preventing Avian Influenza
In a recent study published in the Proceedings of the National Academy of Sciences in the USA, genetic analysis of H5N1 viruses from poultry and birds in China indicated that the virus has been present and spreading in southern China for over a decade. Thus, it can be confidently stated that southern China is the origin of the H5N1 avian influenza outbreak.
Scientists have also shown that migratory birds play a significant role in the dissemination and spread of avian viruses worldwide. In southern China, many healthy poultry are immune but still carry the virus. This is a major problem that needs to be addressed to prevent spreading to uninfected poultry elsewhere. After analyzing the genetic sequences of various H5N1 viruses from wild birds, chickens in the wetlands of Hong Kong, Poyang Lake in eastern China, and chicken and duck markets in southern China, researchers demonstrated, through correlation charts between the viruses, that poultry from southern China infected wild ducks in early 2005.
These wild ducks carried the virus to Poyang Lake in eastern China, and from there it spread to Qinghai Lake, western China, over 1,700 km away, killing many birds in this protected habitat. From Qinghai Lake, the virus was transmitted to Russia and other countries in the Middle East, Mongolia, and Europe.
 |
| Migratory birds play a significant role in the spread of avian viruses worldwide (Photo: cbsnews) |
From southern China, there have been multiple outbreaks of infection into neighboring Vietnam and beyond (Indonesia). In these places, the virus establishes new habitats and exacerbates preventive measures to stop the avian flu from jumping to humans. Therefore, to prevent and control the flu outbreak, it is crucial to first contain the source of the outbreak in southern China.
In Ho Chi Minh City and several provinces, I have noticed many posters and banners informing the public about the dangers of avian influenza potentially spreading to humans and preventive measures, from basic information such as thoroughly cooking chicken meat to warnings that the H5N1 flu, if transmitted to humans, could kill up to 2 million people in Vietnam. Transport vehicles carrying poultry should avoid peak traffic times and follow specific routes. It must be said that Vietnam is actively implementing measures to prevent the avian flu outbreak. The World Health Organization (WHO) has praised Vietnam’s achievements in combating avian influenza and considers it a model for developing countries to follow.
According to the aforementioned study, the genetic analysis of various virus families shows many different types among wild birds and poultry, while previous analyses of viruses in infected humans only revealed two different types. Preventive and therapeutic drugs for one type of virus may be only partially or not effective against another type. Researchers suggest that various different drugs should be developed and stockpiled in advance to be readily available when an outbreak spreads globally among humans.