Neuroscientists have revealed anatomical, chemical, and functional differences in the brains of men and women. These changes occur throughout the brain, in regions associated with language, memory, emotion, vision, and hearing…
 |
|
(Photo: lowculture) |
Researchers are examining how these gender-related variations are associated with differences in perception and behavior between males and females. Their discoveries could indicate tailored treatment paths for both genders regarding conditions like schizophrenia, depression, addiction, and post-traumatic stress disorder.
In January 2005, Lawrence Summers, the President of Harvard University, voiced opinions regarding inherent differences in the brain structures of males and females, suggesting this might be a factor in the relatively low representation of women in science. His remarks hinted at a century-long debate, where some scientists estimated that women’s brains tend to be smaller, defending the notion that females are intellectually inferior to males. However, no one has provided evidence or anatomical differences that could prevent females from achieving excellence in mathematics, physics, or engineering. The brains of males and females have been seen as similar in many respects. Over the past decade, researchers have documented the peculiar manifestations of structural, chemical, and functional changes in male and female brains.
Sex-based anatomical differences suggest distinct disease processing capabilities for males and females. Furthermore, they alert researchers that when exploring brain structure and function, the gender of the subjects must be considered in data analysis, including both males and females in future studies to avoid the risk of obtaining skewed results.
Not long ago, neuroscientists believed that gender differences in the brain were primarily confined to areas responsible for mating behaviors. In a 1966 article published in Scientific American titled: “Sex Differences in the Brain”, Seymour Levine of Stanford University described how sex hormones govern different reproductive behaviors in rats. In the article, Levine only described one brain region, the hypothalamus, a small structure at the base of the brain that regulates hormone production and controls fundamental behaviors such as eating and sex. A generation of neuroscientists believed that “differences in the brain” were mainly related to mating behaviors, sex hormones, and hypothalamic structures.
This perspective has spurred discoveries emphasizing the influence of gender on various cognitive and behavioral areas, including memory, emotions, vision, hearing, facial expressions, and the brain’s response to stress hormones. This advancement has accelerated over the past 5-10 years, thanks to sophisticated, non-invasive imaging techniques such as Positron Emission Tomography (PET) and functional Magnetic Resonance Imaging (fMRI), which allow detailed views into the brains of living subjects. These imaging experiments have revealed anatomical changes in many brain regions.
 |
| (Image: stunning-stuff) |
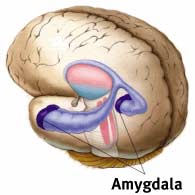
Jill M. Goldstein at Harvard Medical School and her colleagues used MRI to measure the sizes of various cortical and subcortical regions. They found that certain parts of the frontal cortex—associated with numerous cognitive functions—are larger in females compared to males (related to emotional responses). In contrast, in males, certain parts of the parietal cortex, linked to spatial perception, are larger than in females, such as the amygdala—an almond-shaped structure that responds to emotionally charged information through heart rate and adrenaline flow. These size differences are relative and are believed to reflect relative importance in different species. For example, monkeys rely more on vision than olfaction, while the opposite is true for mice. Consequently, the monkey brain allocates larger areas for visual processing, whereas the mouse brain dedicates more space to olfactory functions. Thus, the existence of various anatomical differences between males and females suggests that gender influences how the brain operates.
Other studies are exploring gender differences at the cellular level. Sandra Witelson and her colleagues at McMaster University discovered that females have a higher density of neurons in parts of the temporal lobe associated with language processing and cognition. By counting neurons in postmortem samples, they found that two of the six layers present in the cortex exhibited a higher neuron count per unit volume in females compared to males. With such information, neuroscientists can now investigate whether gender differences in neuron counts correlate with differences in cognitive abilities. Does the increased density in the auditory cortex of females relate to their superior performance on verbal fluency tests?
This anatomical diversity can largely be attributed to the effects of sex hormones permeating the fetal brain. These steroids guide the organization and binding of the brain during development, affecting the structure and density of neurons across various regions. Interestingly, the brain areas where Goldstein observed differences between males and females are also the regions in animals that contain the highest concentrations of sex hormone receptors during development. The correlation between adult brain region sizes and the effects of sex steroids in the womb at least suggests some gender differences in cognitive function that are not due to cultural influence or hormonal changes associated with puberty, but rather are innate.
Innate Trends
Several intriguing behavioral studies provide further evidence of gender differences in the brain that precede a child’s first cry. For many years, researchers have demonstrated that when selecting toys, boys and girls tend to choose differently. Boys favor balls or cars, while girls prefer dolls. However, no one truly believes these preferences are solely driven by cultural influences or innate brain biology.
To address this issue, Melissa Hines at the University of London and Gerianne M. Alexander at Texas A&M University studied monkeys. They allowed a group of long-tailed macaques to choose toys, including rag dolls, pull toys, and some gender-neutral items like books and pictures. They found that male monkeys spent more time playing with “masculine” toys compared to females, while females spent more time playing with items favored by girls. Both genders spent equal time on neutral books, pictures, and other gender-neutral toys.
Since long-tailed macaques are likely not influenced by the social pressures of human culture, these results imply that toy preferences in children are at least partly due to inherent biological differences. This distinction, and indeed the entire scope of anatomical gender differences in the brain, could be a result of selective pressures during evolution.
When Under Stress…
In many instances, gender differences in brain chemistry and structure affect how males and females respond to their environment or stressful events and how they recall them. Returning to the example of the amygdala, Goldstein and other authors noted that the amygdala in males is larger than in females. In male rats, neurons in this brain region exhibit more interconnections than in females. These anatomical changes may create differing responses to stress between males and females, both in animals and humans.
To assess how the amygdala in male and female animals actually responds differently to stress, Katharina Braun and colleagues at Otto von Guericke University in Magdeburg, Germany, quickly removed baby rats from their mothers. They then measured serotonin receptor concentrations in various brain regions (serotonin is a neurotransmitter or signaling molecule primarily mediating emotional behavior). The researchers allowed the baby rats to hear their mothers while isolated and found that serotonin receptor concentrations increased in the amygdala of male pups but decreased in female pups. While it is challenging to extrapolate from this study to human behavior, the results suggest that if similar occurrences happen in children, then separation anxiety may impact emotional well-being differently in boys and girls. Such experiments are crucial if we wish to understand why anxiety disorders are significantly more prevalent in girls than in boys.
Another brain region exhibiting anatomical gender differences and its response to stress is the hippocampus—a crucial structure for memory retention and spatial mapping of the physical environment. Imaging studies have demonstrated that the hippocampus is larger in females than in males. This anatomical difference may relate to how males and females navigate differently. Many studies suggest that males likely rely on estimating spatial distances and orientation, while females depend more on landmarks. Interestingly, similar gender differences are also observed in mice. Male mice navigating a maze tend to rely more on directional information and position, while female mice similarly depend on available landmarks. Moreover, neurons in the hippocampus of male mice also exhibit different expression patterns compared to female mice.
Sometimes, male mice perform better under stress.
 |
||
|
Professor Tracey J. Shors
|
Tracey J. Shors at Rutgers University and colleagues found that a one-second series of tail shocks increased learning performance and dendritic connectivity density with other neurons in male mice, while it decreased performance and connectivity in female mice. These findings have significant social implications. Having uncovered how the brain’s learning mechanisms differ between genders, we may need to reconsider how optimal learning environments should differ for boys and girls.
|
Although female mice showed a lesser response to acute stress, they demonstrated greater resilience compared to male mice under chronic stress. Researchers at the University of Arizona confined mice in a cage for 6 hours. They then assessed how the hippocampal neurons were damaged by a neurotoxin – a standard measure of stress’s effects on these cells. They found that prolonged confinement made the hippocampal cells in male mice more sensitive to the toxin, but it had no effect on damage in female mice. These findings suggest that, in terms of brain damage, female mice may be better equipped to endure prolonged stress compared to male mice. It is still unclear what protects the hippocampal cells in female mice from long-term stress damage, but it may involve the role of sex hormones. In expanding their investigation into how the brain processes and recalls stressful events, Larry Cahill (the article’s author) and colleagues observed a contrast in memory between males and females regarding emotionally charged stimuli (a process known in animal research to be related to the amygdala). In a human experiment, they had volunteers watch a series of violent films while measuring their brain activity using PET scans. Weeks later, when they retested the subjects, they found that the number of films the subjects could recall correlated with the positive activation of the amygdala during the viewing. Subsequent experiments also confirmed this general finding. However, in some studies, amygdala activation was only related to the right hemisphere in males, while in other studies, it was only evident in the left hemisphere in females. To affirm this, the authors used propranolol, a beta-blocker that inhibits the activity of adrenaline and noradrenaline, thereby dampening amygdala activation and reducing the recall of emotionally stimulating memories. Participants in the experiment viewed a brief projection of a teenager involved in a collision while walking with their mother. The memory of the subjects was assessed one week later. The results showed that propranolol made it more difficult for males to remember overarching aspects (the main points), while females focused on the finer details. Along with similar studies, it is suggested that in some healthcare settings, beta-blockers reduce memory of traumatic events in females but not in males, indicating that physicians may need to consider the patient’s gender when prescribing medication. Gender and Psychological Disorders A similar situation is prevalent in addiction cases. In this context, the neurotransmitter involved is dopamine—a chemical associated with pleasure linked to drug abuse. A research group at the University of Michigan discovered that in female mice, estrogen increases dopamine release in brain regions crucial for regulating drug-seeking behavior. Moreover, this hormone has a lasting effect, leading female mice to pursue cocaine weeks after their last drug exposure. Differences in sensitivity, especially to stimulants like cocaine and amphetamine, may explain why females are more vulnerable to the effects of these substances and why they progress from initial use to dependence faster than males. Some brain deficits manifested in schizophrenia also differ between males and females. Researchers at the University of Pennsylvania found that the ratio of the prefrontal cortex to the amygdala was larger in females than in males. These findings suggest that, in general, females may have better control over emotional responses than males. Schizophrenia is a condition that exhibits slight differences between genders, and treating this illness requires consideration of the patient’s gender. In a comprehensive report in 2001 on gender differences in human health, the National Academy of Sciences asserted that: “Gender, meaning male or female, is a fundamental variable that must be considered when designing and analyzing research projects in all fields and at all levels related to biomedical research and health.” Although many issues remain to be examined, research has shown that these differences extend beyond hypothalamic structure and mating behavior. Researchers and physicians are still unclear about the best ways to fully interpret the effects of gender on the brain, behavior, and drug responses. The author of this article is Nguyễn Ngọc Hải – Published in the Scientific Activity Journal, issue 03, 2006 |