60 million, that is the average number of oysters found on a 400,000 square meter oyster reef. This figure may overwhelm you. But it will be even more astonishing if you know that the existence of each of those 60 million oysters is a miracle.
Throughout its life, a female oyster will produce about 100 million eggs. The amount of sperm released by a male oyster is even incalculable, possibly reaching tens of billions.
What’s special is that oysters can alternately change their gender throughout their lives. Sometimes, an oyster is male and releases sperm into the water. At other times, they are female and lay eggs.
Therefore, a reef of 60 million oysters can produce 6 trillion eggs and at least 600 trillion sperm.


When a sperm cell from an oyster combines with an egg, they create a larva. Oyster larvae initially are just tiny soft-bodied organisms measuring less than 1/20 of a millimeter. This fragility means that most oyster larvae will die within 12 hours of being born.
The reason is that oyster larvae have not yet developed a protective shell. On average, after half a day, oysters begin to form their outer shell. They extract calcium from the water and combine these atoms with carbonate groups.
This is how the oyster’s CaCO3 shell is formed. It takes about 3 weeks to build up enough weight for the shell to be heavier than seawater. They pull the soft body of the oyster down to the bottom, where their ancestral reefs await.
From here, the safety of the oyster is ensured.

However, some studies over the past three decades have shown that oysters are taking longer to build their carbonate shells. This is due to the increasing acidification of seawater.
Saltier and more acidic, the ocean is dissolving not only oyster shells but also the shells of many small mollusks and arthropods. The process of ocean acidification is also slowing the growth of many coral reefs, leading to a decline in the diversity of marine organisms that depend on their ecosystems.
Scientists predict that if the pH of seawater drops to a certain threshold, it could cause a mass collapse of marine ecosystems. That is why they rank ocean acidification as one of the nine planetary boundaries, beyond which civilization and life on Earth could be threatened.
The remaining boundaries include: climate change, biosphere integrity, stratospheric ozone depletion, aerosol pollution, freshwater use, biochemical nitrogen and phosphorus flows, land system change, and chemical pollution.
In this article, we will explore why the oceans are becoming acidified? And what consequences will the decrease in seawater pH have?

The ocean is dissolving 22 million tons of CO2 every day
It all started in the 1990s when Ulf Riebesell, a German marine biologist, began studying phytoplankton, a type of microalgae that floats on the ocean’s surface.
These phytoplankton contain chlorophyll and can photosynthesize. They absorb CO2 and release oxygen under the influence of sunlight. This makes phytoplankton a crucial link in the carbon cycle, not only for the ocean but for the entire planet.
Through their biological processes, phytoplankton contribute to atmospheric regulation. They directly produce about half of the world’s oxygen, despite the surprising fact that their total biomass accounts for only about 1% of the total plant biomass on Earth.
Phytoplankton also participate in the food chain, transporting organic and inorganic nutrients as well as trace elements. Thanks to their photosynthetic ability, phytoplankton help fix CO2 from the atmosphere on a global scale.
They recycle carbon and allow it to be reused on the ocean’s surface. A certain portion of the biomass from phytoplankton will become particles that sink deep to the ocean floor, where they continue to undergo transformations, being remineralized into rocks and sediments to store carbon for long periods.

Therefore, looking at this amazing species of algae, Riebesell hypothesized. He thought if phytoplankton thrived through photosynthesis, then when seawater dissolved more CO2, they would naturally grow even more.
However, what Riebesell actually found in the field and in experiments surprised him. Contrary to what Riebesell thought, phytoplankton blooms would wither in seawater that dissolved more CO2.
And so Riebesell began to investigate why? He discovered that phytoplankton have a cell wall made of calcium carbonate. Just like many calcifying marine animals, water with higher CO2 levels would hinder the calcification process of phytoplankton and threaten the survival of this algae.
In 2004, Riebesell brought his findings to a symposium in Paris, where he met many other colleagues who were also conducting similar research on the phenomenon of CO2 absorption into seawater.
Previously, scientists thought that the ocean absorbing CO2 was a good thing. Because if the ocean swallowed CO2, the amount of greenhouse gases in the atmosphere would decrease. Our planet would be less heated, and the lives of terrestrial organisms would be less harsh.
However, what Riebesell and other scientists found showed that the ocean was paying the price for its benevolence. “That was the moment when suddenly, our community realized that the ocean’s pH imbalance could be harmful. That was also when the term ocean acidification was born,” he said.

Ocean acidification is defined as the decrease in pH of the entire ocean or certain sea areas on Earth. This phenomenon can occur on a local scale due to several reasons such as changing ocean currents, volcanic activity, or a decrease in sea ice density.
However, on a global scale, the primary cause of ocean acidification is the absorption of carbon dioxide (CO2) from the atmosphere into seawater.
Scientists state that in just 200 years since the industrial revolution, humans have emitted 2.3 trillion tons of CO2 into the atmosphere, primarily through fossil fuel extraction and combustion. This has pushed greenhouse gas levels in the atmosphere to the highest levels in the past 15 million years.
CO2 in the atmosphere slows down the rate at which Earth loses heat to space. Instead, it traps heat, creating a layer of warm air and gradually warming the Earth’s surface over time. This is the underlying cause of global warming and climate change, which is causing increasingly extreme and unpredictable weather patterns today.
Things would be even worse than what we are currently seeing if the oceans did not participate in the carbon cycle, where seawater acts like a membrane stretched over 70% of the Earth’s surface area, ready to absorb up to a quarter of the greenhouse gases produced by humans.
As a result, since the early industrial era, the world’s oceans have absorbed over 500 billion tons of CO2 from the atmosphere. This figure is equivalent to 22 million tons of greenhouse gases each day.
Initially, scientists thought that the ocean, by itself, could solve its own CO2 problem. They expected that when freshwater flows dissolve the lithosphere and pour into the ocean, the base ions they carry could balance out the rate of seawater pH decline.
This is scientifically referred to as the buffering effect, which has kept seawater’s pH stable at around 8.1-8.3 for millions of years. But it turns out that humans can disrupt any equation that Mother Nature has balanced.
Riebesell’s discoveries and those of his colleagues in the 2000s showed that in the face of strong CO2 absorption from the atmosphere, the buffering effect could no longer maintain the balance of seawater pH.
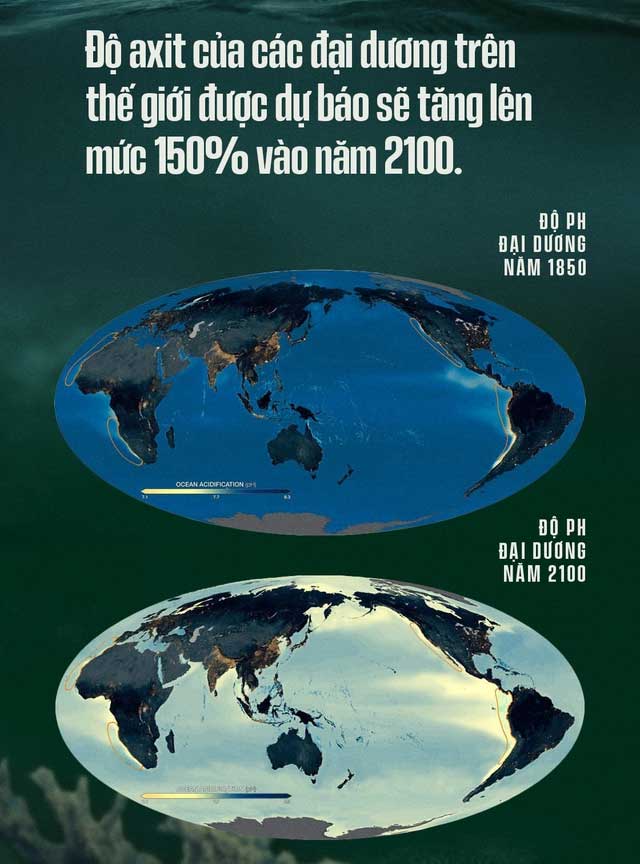
The result is that in just 200 years after the industrial revolution, the pH of the ocean has decreased from 8.25 in 1751 to 8.14 today. Although the change is only 0.1 units on the pH scale, because pH is a logarithmic function, each unit represents a tenfold change in H+ ions.
Scientists report that a significant phenomenon is occurring: the ocean waters of all the Earth’s oceans have seen an increase in H+ concentration of about 30%. In just the last 200 years, this represents a rapid increase that is 100 times faster than the natural chemical changes that occurred in the oceans over the past 50 million years, prior to human existence.
Chemical Equation of the Oceans
It should be noted that the oceans have become 30% more acidic compared to pre-industrial times, which does not mean that all seawater has turned into acid. In fact, on the pH scale from 0 to 14, the current pH of seawater still shows mild alkalinity, at a value of 8.14.
Scientists estimate that even if all the CO2 in the atmosphere were to dissolve into the oceans, it would not be enough to pull the pH of seawater below 7, meaning it would not actually become acidic.
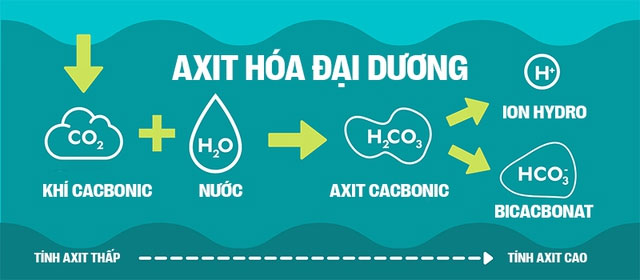
However, what they want to emphasize in the concept of “ocean acidification” is the increase of hydrogen ions (H+) in seawater. After CO2 dissolves in water (H2O), it forms a weak acid known as carbonic acid (H2CO3).
H2CO3 is unstable and will dissociate into H+ ions and bicarbonate (HCO3-). The reaction can even proceed further, as bicarbonate continues to dissociate into an H+ ion and a carbonate ion (CO3 2-).
The increase of these H+ ions creates problems for marine organisms that have adapted to the alkaline environment of the oceans for millions of years.
We know that many chemical reactions, including those necessary for life, are very sensitive to small changes in pH. For example, in humans, normal blood pH ranges from 7.35 to 7.45. A drop in blood pH by 0.2-0.3 units can cause seizures, coma, and even death.
Similarly, a small change in the pH of seawater can have significant impacts on marine organisms, affecting biochemical processes, growth, and reproduction.
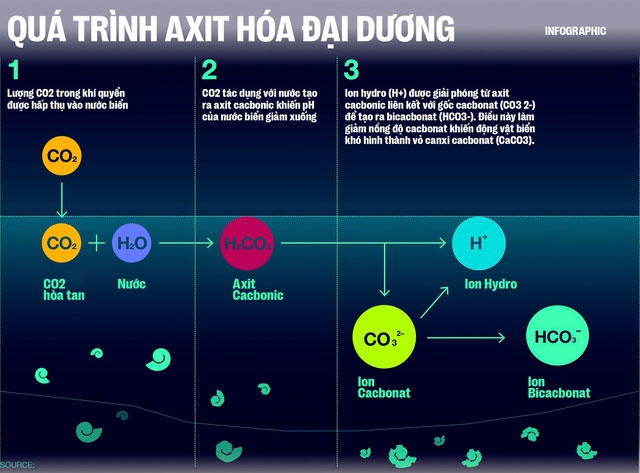
Earlier, you heard about oyster larvae and phytoplankton, but the truth is that many other marine organisms are also building their shells or skeletal frameworks from calcium carbonate (CaCO3). To do this, these organisms need to combine calcium ions (Ca2+) with carbonate (CO32-) from the surrounding seawater. This process releases CO2 and water.
However, H+ ions, which have a stronger attraction to carbonate (CO32-) than to calcium ions, hinder the process of shell and skeletal framework construction for marine organisms.
Even if the number of H+ ions is small enough for marine organisms to still synthesize calcium carbonate, they will expend more energy and time to do so. When these species focus their resources on survival, they must pay the price with a limited reproductive process. As a result, their populations will decline.
Worse still, if there are too many hydrogen ions present, the calcium carbonate shells and skeletal frameworks of marine organisms may even dissolve. Scientists warn that at the current rate of ocean acidification, the pH of seawater could drop an additional 0.3 to 0.5 units, reaching levels as low as 7.8 by 2100.
At this level of acidification, experiments have shown that seawater could dissolve the shells of pteropods, a small marine snail, in just 45 days, exposing this fragile organism and threatening its survival.

Species with thicker carbonate shells, such as snails, clams, shrimp, crabs, sea urchins, and starfish, will also be thinned out, making them more vulnerable to predators.
For instance, one study found that the shells of mussels would dissolve by about 10% in the seawater of 2100. The figure is 25% for oysters, if they can develop and survive through their larval stage.
In fact, ocean acidification has led to numerous mass die-off events of oysters worldwide. In Willapa Bay, Washington, the Puget Sound area, and off the coast of Vancouver Island, wild oyster beds have been reported as no longer capable of reproduction.
Oyster farms in Netarts Bay, Oregon, USA, recorded larval mortality rates of up to 80% around 2006-2008. The initial cause was attributed to bacteria, but even after these farms spent thousands of dollars to filter and sterilize the water, their oyster larvae continued to die.
Ultimately, only when scientists stepped in and pointed out that the pH of seawater was the reason oyster larvae could not form shells, was the $73 million oyster industry in the Pacific Northwest saved. They are now required to mix sodium bicarbonate in their oyster beds to counteract the extreme acidity of seawater.

The next significant impact of ocean acidification is placed on a special organism: coral. Being an invertebrate, coral also relies on the process of synthesizing calcium carbonate from seawater to form a skeletal structure that serves as a home for the resident polyps.
Scientists indicate that ocean acidification can affect corals even before they build their homes. Similar to oysters, the eggs and larvae of some coral species cannot mature and find a habitat in environments with excessive H+ ions.
Even if some coral species can overcome that challenge, they will continue to struggle as seawater erodes their skeletal structure. Calculations indicate that from now until 2080, seawater will become acidic enough to dissolve more coral than they can accumulate.
The result will be a phenomenon known as coral bleaching, where coral reefs completely lose the symbiotic algae living on them, leaving only the stark white carbonate skeletons behind.
Bleached corals, although still alive, will be more susceptible to disease and death, leading to the decline of an entire ecosystem that serves as a home for thousands of other marine organisms.

Fish are the next victims of acidified seawater. Most visibly affected are those fish species that rely on coral when they lose their homes. More subtly, the change in seawater pH also impacts many biochemical and neurological processes in fish.
For example, clownfish are believed to become more disoriented in acidified seawater. Excess H+ ions impair their ability to “smell” their way home.
Fish hearing and navigation abilities are also affected by acidified seawater. Many fish species, such as clownfish and grouper, can normally hear predators, but when seawater pH decreases, their hearing ability also diminishes.
Moreover, fish face an opportunity cost when living in an acidified environment. Even without carbonate shells or skeletons, fish cells typically create a pH balance with seawater by absorbing carbonic acid.
To prevent their blood from becoming acidic, fish must maintain biochemical processes in their gills, kidneys, and intestines to excrete acid. Thus, the lower the pH of seawater, the more energy fish expend to do so.
The result is that the energy they spend on foraging, digestion, reproduction, and escaping predators will decrease. Ocean acidification is predicted to slow the growth of many fish species, leading to a decline in their populations.

What Could Be the Worst Outcome?
At this point, you might imagine that ocean acidification is similar to inflation in human society. Just as inflation increases our cost of living, a decrease in pH makes life more challenging for many marine species.
The loss of any one of these species will also impact the ocean’s food chain and potentially cause a collapse. Scientists have looked into Earth’s history to understand what the worst possible outcomes could be.
They do this by drilling down to the ocean floor and collecting rock samples deep within the planet’s crust. The chemical composition of these fossil samples reveals that about 55.8 million years ago, the oceans on Earth experienced a level of acidification similar to what we see today.
The cause was excessive volcanic activity that burned down forests, causing widespread wildfires and releasing a large amount of CO2 into the atmosphere. Earth’s temperature at that time rose by 5 degrees Celsius, marking the period known as the Paleocene-Eocene Thermal Maximum.
The sedimentary layers found to represent this period are characterized by a reddish-brown color of mud instead of the chalky white of calcium carbonate. This serves as evidence that marine organisms with shells have been dissolved by acidified water.

Previous studies have shown that ocean acidification has contributed to three of the five largest mass extinctions in Earth’s history. Initially, scientists believed that these mass extinctions primarily occurred on land due to excessively high concentrations of CO2 in the atmosphere.
However, research from 2004 indicated that ocean acidification also triggered mass extinction events beneath the ocean floor at the end of the Triassic, Permian, and Cretaceous periods.
Today, humanity is facing its own era of ocean acidification, as the amount of CO2 we release into the atmosphere is unprecedented in both volume and speed. Human industrial activities have generated more CO2 than during the Paleocene-Eocene Thermal Maximum.
Currently, atmospheric CO2 concentrations have surpassed 400 parts per million (ppm) – while the safe level is about 350 ppm, a critical threshold we have exceeded since 1988. Without ocean absorption, atmospheric CO2 levels could reach as high as 475 ppm.
Therefore, we truly do not know what will happen as our civilization continues to emit and the oceans continue to absorb CO2 into seawater.
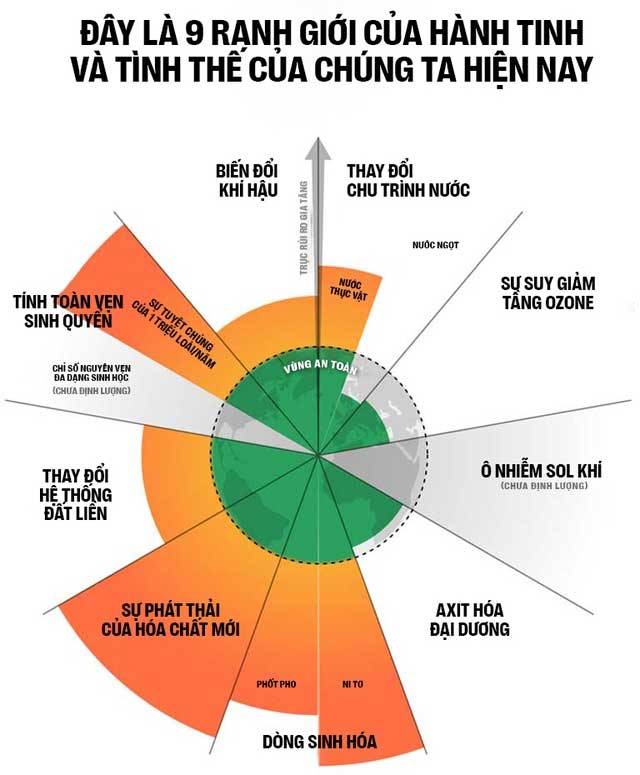
Some scientists have contemplated a boundary, a threshold of ocean acidification that, if crossed, would lower the pH of seawater to such an extent that it threatens the functioning of the entire ecosystem on Earth.
Ocean acidification is classified as one of the nine planetary boundaries, which include: climate change, biosphere integrity, ozone layer depletion, aerosol pollution, freshwater use, biochemical nitrogen and phosphorus flows, land system change, and the release of novel entities.
A study published in 2015 indicated that humanity has already crossed four boundaries: climate change, biosphere integrity, land system change, and biochemical nitrogen and phosphorus flows.
More recently, scientists pointed out that we are also entering the danger zone of two additional boundaries: the release of novel entities, specifically microplastic pollution, and the destabilization of the freshwater cycle.
Fortunately, ocean acidification is still within a safe threshold. However, that threshold will be surpassed if the pH of the ocean drops to the level of aragonite dissolution – an important form of calcium carbonate that many organisms use in their calcification processes.

Once this boundary of ocean acidification is breached, we will enter a period of extreme uncertainty.
To prevent this from happening, the most practical action we can take is to reduce emissions and enhance carbon fixation processes on the planet. Emission reductions can be achieved by decreasing fossil fuel usage. Effective carbon fixation measures include reforestation, restoring seagrass beds, and cultivating more algae.
Although these measures require macro-level international efforts to be effective, you can also contribute to preventing ocean acidification through simple actions.
For example, turn off lights when not in use, walk or bike for short distances instead of using motorbikes or cars, utilize public transportation, and harness renewable energy from solar, wind, or geothermal sources…
Even checking your tire pressure can help reduce gasoline consumption, thereby lowering your personal carbon emissions.
Additionally, one more thing you can do is share this article to inform friends and family about the acidification of our oceans. Even scientists have only discovered this issue in the last two decades, and many people are still unaware that our seawater is becoming more acidic.
The consequence of this is that the face of the ocean will change, coral reefs will disappear, shells will thin, oysters will vanish, and even our civilization will be pushed to a dangerous boundary.