A new study highlights an unprecedented mechanism of transmission: the SARS-CoV-2 virus creates nanotubes to spread from the nose to nerve cells.
The SARS-CoV-2 virus primarily targets the respiratory system, but in some cases, it also affects other organs such as the intestines, liver, kidneys, heart, and brain.
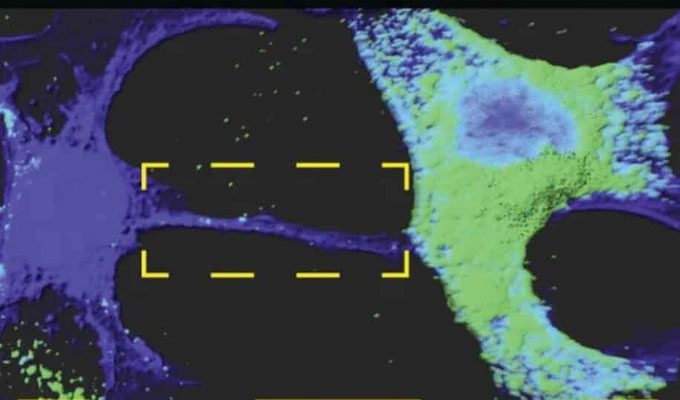
Nanotubes allow SARS-CoV-2 virus to move into nerve cells. (Photo: Anna Pepe).
How the virus enters the brain and causes neurological symptoms remains unclear because the main “gateway” of the virus – the angiotensin-converting enzyme 2 (ACE2) receptor – is hardly detected in brain cells (unlike in nasal and lung cells).
Understanding how the SARS-CoV-2 virus infiltrates nerve cells is key to comprehending (and treating) the neurological manifestations associated with Covid-19.
Chiara Zurzolo, research director at the Pasteur Institute, and her team discovered that the COVID-19 virus has an entirely different pathway to invade cells lacking ACE2 receptors.
Specifically, it exploits a communication pathway between cells known as “tunneling nanotubes” (TNT) or “tunneling nanotube effect.”
Direct Transmission Pathway of the Virus
TNTs are tiny tunnels, measuring a few dozen nanometers in diameter, that allow at least two cells to exchange ions, entire organelles… and even viruses! In 2016, Chiara Zurzolo’s team demonstrated that TNTs play a role in the intercellular spread of pathogenic amyloid proteins associated with Alzheimer’s and Parkinson’s diseases.
Scientists have continued to study the activity of these nanotubes since then to clarify their involvement in the transmission of certain viruses and bacteria within the body.
Faced with the neurological consequences of SARS-CoV-2 infection, the research team hypothesized that the virus could also use TNTs to spread from easily infectable cells to less permissive cells (those lacking ACE2 membrane receptors) while simultaneously evading immune surveillance.
To test this hypothesis, they cultured nerve cells (which do not allow infection through the intracellular route) in the presence of infected epithelial cells (which do allow infection).
By examining their cultured cells under a microscope with the same specimen, the experts discovered that nerve cells could be infected via the TNT-mediated mechanism when co-cultured with permissive epithelial cells.
“After 24 hours of co-culture, 36.4% of the receiving cells contained signals recognized by antibodies against N (note: antibodies specific to the N protein of SARS-CoV-2) in their cytoplasm, and this rate increased to 62.5% after 48 hours,” the researchers reported.
Additionally, they found that the SARS-CoV-2 virus present in the epithelial cells (representatives of the cells lining the nasal passages) stimulated the formation of nanotubes to establish connections with existing nerve cells (which the virus could not infect on its own).
Subsequently, it redirected these cells away from their original functions, such as lipid and protein transport, to overwhelm them.
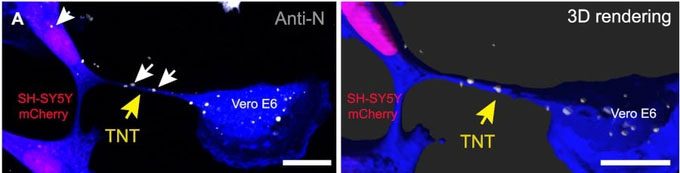
Infected Vero E6 cells co-cultured with SH-SY5Y cells. Yellow arrows indicate TNT; white arrows indicate anti-N signals of SARS-CoV-2 inside the TNT and in the receiving cells (Photo: Anna Pepe).
Thanks to the magnification capabilities of modern observational equipment, scientists were able to observe the virus transferring from one cell to another.
According to the research group, the replication sites of the virus observed within the TNTs between permissive and non-permissive cells also revealed the proteins linked to the cellular machinery that the virus uses for replication.
In summary, not only has the SARS-CoV-2 virus found a way to infiltrate cells that it could not access via the classical route, but this mechanism also enhances the virus’s spread among permissive cells, in addition to the intracellular pathway.
This is not the only virus that controls cells in this manner: HIV and influenza viruses also exploit TNTs to move from one cell to another. These nanotubes may also be the source of long Covid hidden within, and the virus can indeed evade antibodies and persist longer in the human body.
This experiment was conducted in vitro, on cell models, and further studies will be necessary to confirm that this mechanism also operates in the human brain.
If this operational method is validated, Zurzolo’s research team suggests that this could lead to the development of drugs capable of preventing the formation of nanotubes or severing them.


















































