New Biocompatible and Water-Soluble Implantable Devices May Replace Opioid Painkillers and Other Addictive Medications.
Researchers at Northwestern University have developed a small, soft, and flexible implantable device that can alleviate pain on demand without the need for drugs or solvents. According to the research team, the device is highly valuable for patients undergoing routine surgeries or amputations who require post-operative pain management. Surgeons can implant the device during the procedure to help relieve the patient’s pain after surgery. The study, published on July 1 in the journal Science, describes the device’s design and its effectiveness in animal models.
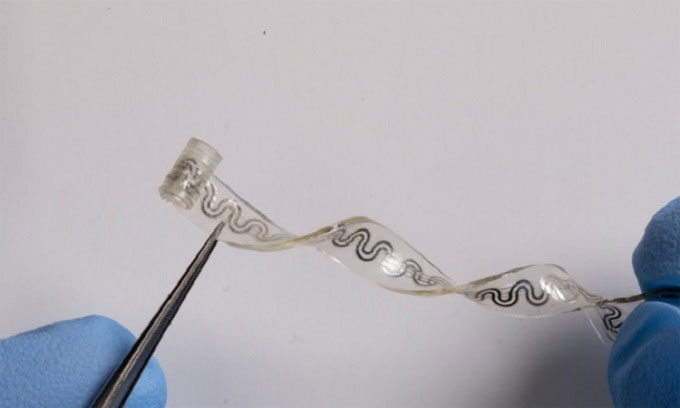
Flexible implantable device easily stretches and bends inside the body. (Photo: Northwestern University)
“While opioid medications are highly effective, they also have a high potential for addiction,” said John A. Rogers, the lead researcher at Northwestern University. “As engineers, we are driven by the idea of treating pain without the use of drugs in a way that can be activated or deactivated immediately. In animal models, our implantable device demonstrates that this effect can be pre-programmed, targeting local nerves and even those surrounding soft tissues.”
The implantable device is based on a simple concept: evaporation. It contains a cooling liquid that evaporates at specific locations on sensory nerves. The device works by wrapping around the nerve, providing precise and targeted cooling effects. This paralyzes the nerve and prevents pain signals from reaching the brain. An external pump allows users to activate the device remotely and control its intensity. Once the device is no longer needed, it is naturally absorbed by the body, eliminating the need for surgical removal. With a thickness comparable to a sheet of paper, the elastic device is ideal for treating highly sensitive nerves.
Dr. Matthew MacEwan from the Washington University School of Medicine in St. Louis noted that as the nerve cools, the signals transmitted through it slow down and eventually stop completely. “We target peripheral nerves that connect the brain and spinal cord to the rest of the body. These are the nerves that convey sensory stimuli, including pain. By providing effective cooling to one or two nerves, we can modulate the pain signal in a specific area of the body,” MacEwan explained.
The device contains tiny microchannels that transmit the cooling effect. While one channel holds the cooling liquid perfluoropentane, the other contains dry nitrogen. When the liquid and gas flow into a common chamber, a reaction occurs that causes the liquid to evaporate instantly. Integrated micro-sensors monitor the temperature of the nerve to prevent it from becoming too cold, which could cause tissue damage.


















































