Humans possess a unique sequence of two amino acids in the FOXP2 gene located on chromosome 7. This FOXP2 gene regulates the development of critical brain structures necessary for speech movements, enabling us to communicate verbally.
Among all the abilities that define human uniqueness, the capacity for speech stands out prominently. This ability differentiates humans from other species, including our closest evolutionary relatives, the apes, who lack this capability.
Detailed studies of genetic material and fossils have dated the evolutionary divergence of humans from chimpanzees (genus Homo and Pan) to approximately 7-8 million years ago (as illustrated in the family tree below). Due to their close ancestral ties to humans, scientists have been eager and curious to explore the linguistic abilities of chimpanzees.
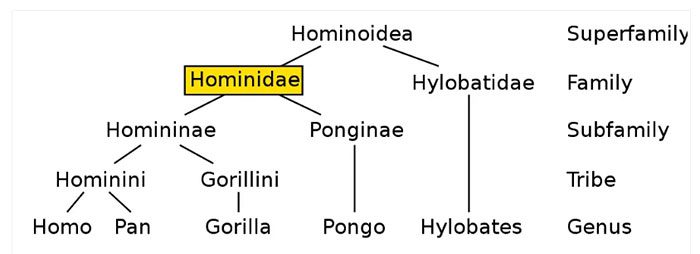
The Hominidae family tree shows humans (Genus: Homo) diverging from chimpanzees (Genus: Pan) approximately 7-8 million years ago
Specifically, in a recent project, researchers attempted to teach sign language to a chimpanzee named Nim Chimpsky (named after Noam Chomsky!), who was raised in a human social environment. Surprisingly, despite persistent and close training, Nim was unable to combine words using grammatical rules. In contrast, deaf children with minimal exposure to spoken language can easily express grammar through their sign language. This raises the question: if chimpanzees are indeed closely related to us, why is it that only humans can speak? What secrets does human genetic material hold that enable us to engage in such remarkable conversation?
With the question “why is speech a unique ability of humans”, the first clue emerged when scientists studying language disorders stumbled upon a unique case. They discovered a family (referred to as the KE family) where approximately half of the members experienced severe language and speech disorders over three generations. These individuals exhibited a disorder in coordinating facial muscles while speaking, known as “developmental coordination disorder” or “specific language impairment”. This made it difficult for listeners to understand what they were trying to express. Furthermore, affected members showed difficulties adhering to grammatical rules. The ability to move facial muscles for speech and to follow grammatical rules has set humans apart from other primates. This is a noteworthy study.
Curious about the genetic basis of the impairments in the KE family, researchers conducted genetic analyses. They found that both males and females in the KE family were equally affected, leading them to focus on autosomal (non-sex-linked) chromosomes. The KE family tree, displayed below, shows affected members (in black). Scientists suspected that this disorder was due to a region on chromosome 7, found in all 27 affected members. They named it the “SPCH1” region. However, this region contains about 70 genes, and it was unclear which gene was responsible for the specific language impairment.
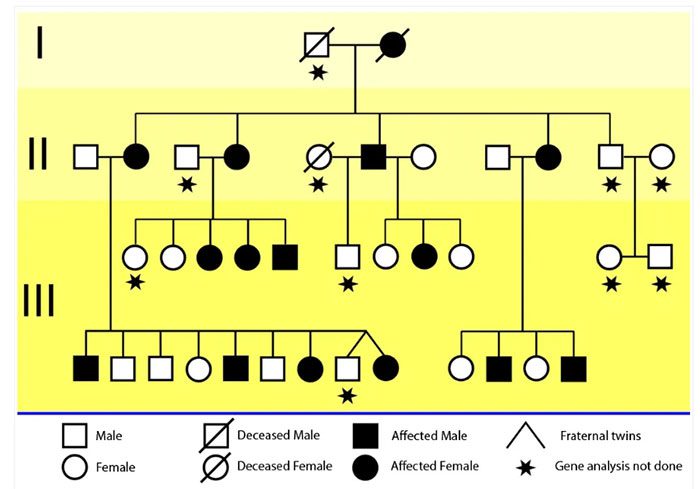
The KE family tree across three generations. Circles represent females, and squares represent males. Black shapes indicate members affected by speech function.
A subsequent report from an unrelated individual regarding similar speech difficulties as those in the KE family helped researchers continue their investigation. Genetic analysis of this affected individual revealed a break in chromosome 7, between a gene known as “FOXP2” (Forkhead Box P2). Scientists now know that the FOXP2 gene in the SPCH1 region is the culprit behind this condition.
The next part of the puzzle linking FOXP2 and speech comes from analyzing the genetic code within the gene and determining how it has changed over the course of evolution. Researchers traced the evolutionary origins of the FOXP2 gene by comparing it across species—mice, rhesus monkeys, orangutans, gorillas, chimpanzees, and humans. Interestingly, this is an extremely “conserved” gene, with all FOXP2 proteins from chimpanzees, gorillas, and rhesus monkeys differing by only 2 (out of 715) amino acids from the human FOXP2 protein. Furthermore, humans, across all different regions compared, all showed that the FOXP2 gene encodes for two uniquely identical amino acids.
The FOXP2 gene is present in all animal species, yet none can speak except for humans. This leads experts to believe that the unique changes in those two amino acids might play a crucial role in human speech capabilities.
Surprisingly, despite the differences in coding genes, FOXP2 has been shown to have similar functions across several animal species. A detailed study of the FOXP2 gene in mice revealed its important role in the proper development of brain cells in the fetus, which later are responsible for the motor skills of mice. Researchers also created “animal models” with mutated FOXP2 genes in mice and songbirds that mimic the KE family.
These mice exhibited severe deficits in learning motor tasks and reduced ability to vocalize. The mutated songbirds were unable to learn how to pronounce or sing their own “songs,” and they could not even mimic properly when taught by a “tutoring bird.”
All this significant evidence has led experts to believe that FOXP2 plays a crucial role in controlling the articulatory motor functions of species.
Considering that humans possess a unique amino acid sequence encoded by the FOXP2 gene, it is essential to explore its function in humans. It would be inaccurate and unfair to extrapolate findings from other animals with different FOXP2 sequences to explain its function in humans. To investigate the impact of the FOXP2 gene on human brain function, scientists scanned the brains of affected members of the KE family while they performed various language tasks and compared them with unaffected family members. The results indicated that in affected members with speech function, their Broca’s area exhibited reduced activity—this is the brain region critical for our ability to speak. Damage to this area in humans results in an inability to speak, known as aphasia.
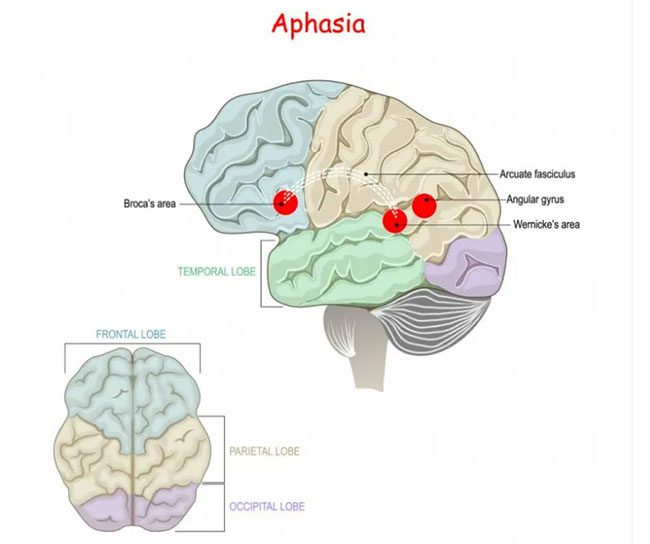
FOXP2 mutation affects the activity of Broca’s area, a motor region associated with aphasia.
Other studies have also indicated that FOXP2 regulates the function of several other genes, so when disrupted, it can lead to language disorders, such as autism spectrum disorder (ASD) and specific language impairment (SLI). These findings suggest that the expression of the FOXP2 gene is crucial for the normal functioning of human brain regions that underpin our ability to speak.
Although linguistic ability in humans is considered a complex skill that genes cannot solely control, the truth is that much of this capability has been endowed to us thanks to the presence of a unique sequence in the FOXP2 gene on chromosome 7. The FOX family of genes acts as regulators of several other genes, “turning them on and off” when necessary. This regulation also leads to the proper development and functioning of the neurons that serve our speech capabilities, including those in the Broca’s area.
It is also important to note that FOXP2 is not a “single gene” responsible for speech. Several other genes controlled by FOXP2 also contribute to our linguistic abilities. However, FOXP2 is “the highest” in the hierarchy of genes controlling the development of brain structures crucial for the fine movements of the mouth and face necessary for speech. It is the only gene sequence uniquely expressed in humans, differing from chimpanzees by just two amino acids, allowing us to better control our mouth movements.
This is also the reason we differ from other species, both apes and chimpanzees—our closest living “relatives.”




















































