A recent discovery could revolutionize battery technology and change the way we power the world.
For decades, scientists have been striving to find the best technology to power modern life while ensuring ecological balance, practicality, and environmental protection. The answer may have been right in front of us: Sulfur.

Sulfur could solve the battery problem that has puzzled scientists for decades (Image: Getty).
However, due to technological limitations, since the early 1990s, the chosen battery technology has been lithium-ion. Since then, lithium-ion has powered everything from smartphones, laptops, and electric vehicles to backup power grids and even space satellites. However, this material has some relatively serious downsides.
Lithium-ion Batteries: Outdated but Still the Only Option
When discussing the drawbacks of lithium-ion batteries, the first issue is cobalt—a material used in batteries that is harmful to our ecosystems, contributing to the destruction of vast ecosystems and even releasing toxic chemicals.
Moreover, lithium-ion batteries also face issues with battery lifespan, as they quickly lose their original capacity after multiple charges (often referred to as “battery aging”). This forces consumers to incur additional costs to replace batteries if they don’t want their devices to become useless.

Lithium-ion batteries still have many drawbacks.
Lithium-ion batteries are also quite heavy, large, and bulky. This limits the operational range of electric cars because the batteries are very heavy, and makes them impractical for certain applications, such as commercial electric aircraft or ships.
Furthermore, lithium-ion batteries are prone to incidents that can lead to fires or explosions; just one damaged cell can cause the battery to ignite and burn violently.
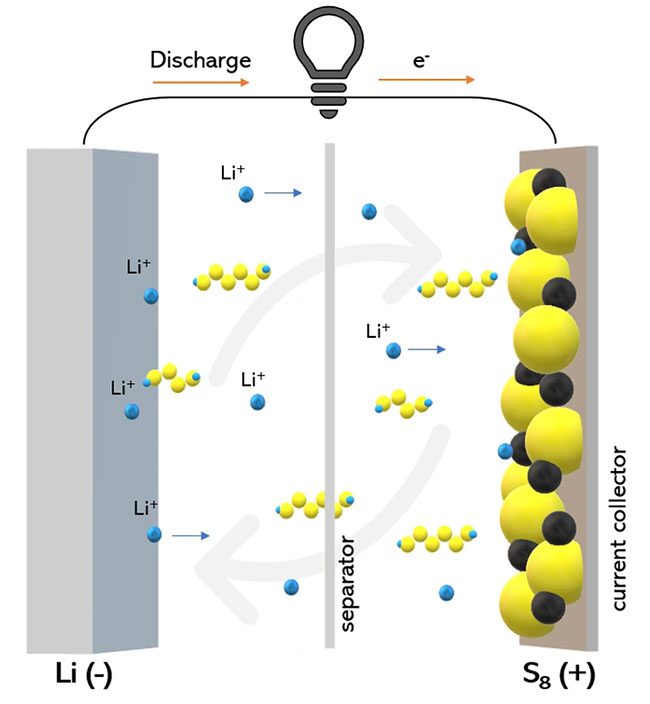
This is why a group of scientists at Drexel University in Germany is researching a completely new type of battery called lithium-sulfur (or sulfur battery).
On the surface, using sulfur in lithium-sulfur batteries could solve all the issues associated with lithium-ion. They utilize less toxic materials ecologically, are cheaper to produce, but ensure a higher energy density—up to three times greater—and are less likely to catch fire.
However, why has lithium-sulfur not yet been chosen to replace lithium-ion? The reason lies in a significant problem: the charge cycle.
While lithium-ion batteries can be used for about 2000 charge cycles, lithium-sulfur batteries are typically limited to only about half that. Therefore, after approximately 1 or 2 years of proper use, lithium-sulfur batteries will essentially “die.”
Sulfur Batteries: Lower Production Costs and Three Times Higher Energy Density
To address this issue, the research team has been experimenting with new approaches to lithium-sulfur by altering the compounds in the battery’s anode.
Their goal is to slow down the chemical reaction that produces polysulfide when charging the battery and storing electrical energy. This reaction effectively extracts sulfur from the electrode, allowing the batteries to become very energy-dense and last longer.
New research on sulfur batteries could change the “game” in the energy sector.
Previously, this chemical reaction was referred to as “monoclinic gamma-phase sulfur”, but it had only been observed in laboratories at high temperatures—up to 95 degrees Celsius.
However, in practical experiments under normal conditions, this reaction yielded surprising results, completely halting the reaction that produces polysulfide. It was so effective that scientists charged the battery through 4,000 cycles without any loss in capacity. This means it lasts at least twice as long as lithium-ion.
Nevertheless, like many other serendipitous discoveries, scientists still do not fully understand what has actually happened. Why does this phase of sulfur appear at room temperature, and how can it be replicated?
Thus, further research is needed to answer these questions and to develop this concept into a stable battery.
If this technology is successfully implemented, it could make the dream of a lightweight, high-capacity, long-lasting, and inexpensive battery a reality, giving electric vehicles a significant advantage over conventional fossil fuel-powered vehicles.
Moreover, lithium and sulfur are abundant elements on Earth. Therefore, using them in electric batteries would significantly reduce the environmental impact during extraction and ensure a more robust supply chain.


















































