Researchers at Pennsylvania State University have developed a blood glucose monitoring device that uses sweat. This is considered the world’s first non-invasive, wearable blood glucose monitoring device.
Detecting Low Glucose Levels in Sweat

A conventional blood glucose meter available on the market.
Currently, non-invasive blood glucose monitoring devices are not available for sale in the U.S., meaning that individuals with diabetes must take blood samples or use sensors implanted under the skin to monitor their blood sugar levels. Thanks to this new device, non-invasive blood glucose monitoring could become the standard.
The research team is led by Professor Huanyu Larry Cheng from the Department of Engineering Science and Mechanics at Pennsylvania State University. They have published details about the low-cost, non-invasive sensor device that can detect glucose in sweat using sensors and bioelectronics.
Initially, the researchers created this device using laser-induced graphene (LIG). This material consists of thick layers of carbon in various shapes. With high electrical conductivity and a fabrication time of just a few seconds, LIG appears to be an ideal framework for sensor devices.
However, Professor Cheng noted that a challenge exists because LIG is not sensitive to glucose at all, requiring the addition of a glucose-sensitive material to the LIG.
According to Cheng, the research team chose nickel due to its strong sensitivity to glucose and combined it with gold to reduce the risk of potential allergies. The researchers hypothesized that LIG equipped with a nickel-gold alloy could detect low glucose levels in sweat on the skin’s surface.
The glucose-sensitive material is a priority. Sweat exhibits significantly lower glucose concentrations compared to blood. Yet, as Professor Cheng points out, there is a close correlation between sweat and blood.
Although the glucose concentration in sweat is approximately 100 times lower than that in blood, the researchers’ device is sensitive enough to accurately measure glucose levels in sweat and reflect those concentrations in the blood.
The sensitivity of the nickel-gold alloy allows Professor Cheng’s team to eliminate the enzymes typically used to measure glucose in more invasive commercial devices. However, these enzymes can degrade quickly over time and with temperature changes.
Professor Cheng explained that an enzymatic sensor must be maintained at specific temperature and pH levels and cannot be stored for long periods. In contrast, the enzyme-free glucose sensor has the advantage of stable performance and glucose sensitivity regardless of these changes.
Enhancing the Device for Broader Applications
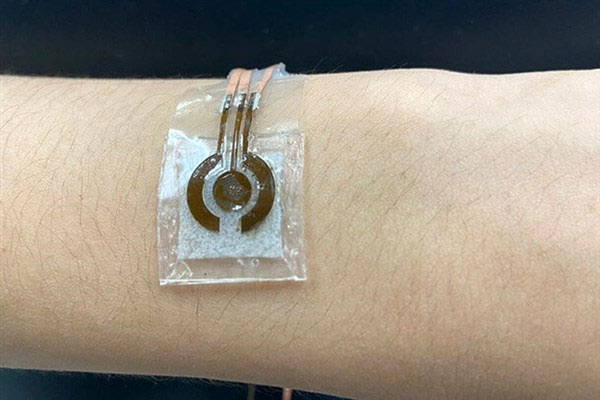
The non-invasive blood glucose monitoring device developed by researchers at Pennsylvania State University.
However, the enzyme-free sensor requires an alkaline solution, which can easily damage the skin and limit the device’s wearability. To address this issue, Professor Cheng and his colleagues attached a microfluidic chamber to the LIG alloy.
This chamber is smaller than previously developed configurations to enhance durability and porosity, allowing for a range of movements such as stretching or crushing. It is connected to a component that delivers sweat into the solution without allowing the solution to contact the skin.
The alkaline solution interacts with glucose molecules to produce a compound that reacts with the nickel-gold alloy. This reaction triggers an electrical signal indicating the glucose concentration in sweat.
According to Professor Cheng, with such a small alkaline solution chamber, the device has enough flexibility to maintain a secure attachment to the human body.
In one trial, researchers used skin-safe adhesive to attach this reusable device to a person’s arm for 1 hour and 3 hours after a meal. The subject performed a short workout sufficient to induce sweating before each glucose measurement.
Minutes after collecting sweat, researchers observed that the detected glucose concentration decreased from the first measurement to the next. The blood glucose measurements from the device were then validated using a commercially available glucose meter.
Professor Cheng and his team plan to enhance this device for future applications, including improving how patients and doctors can use the sensor to measure rising blood glucose or monitor to determine treatment measures, such as insulin use.
They also intend to refine and expand this platform to monitor other biomarkers that may be found in sweat.
| “Researchers want to work with doctors and other healthcare providers to explore how to apply this technology for daily patient monitoring. This glucose sensor serves as a prime example of how the ability to detect biomarkers in sweat at extremely low concentrations can be improved,” said Professor Huanyu Cheng. |


















































