With the simple principle that you can generate electricity from a lemon.
Power Sources
Electricity sold to households by the government is alternating current. We also use electricity in the form of direct current, such as batteries and cells. Batteries are used as power supplies for cars and motorcycles, while cells are compact devices that are very familiar to us, found in watches, TV remotes, etc.
But why do we always think of buying batteries from the store? Can we create a small direct current source ourselves for some daily activities? For example, for a night light… We can easily and safely generate electricity similar to that of batteries.
Basic Components of a Battery
To understand the phenomenon of electromotive force (which is the voltage) and the current generated from a lemon, we need some simple materials as shown.

Materials used to make a lemon battery.
First, insert two pieces of wire or metal into the lemon, making sure they do not touch each other. To check if there is a voltage between these two metals, connect the two metal wires to a multimeter.

First, insert two pieces of wire or metal into the lemon.
What happens if the two metal wires used are the same (two copper wires, two zinc wires, or two screws)? There will be no voltage generated, right? Now, try using one copper wire and one screw, or one copper wire with a paper clip. Does the multimeter show about 0.8 – 0.9V? This means there is a voltage generated between these two metals.

What happens if you squeeze lemon juice into a cup and place two different metal wires in it?
Now, squeeze lemon juice into a cup, then dip two metal wires into the cup (ensuring they touch the lemon juice and that the two metals touch each other). Connect these two metals to the multimeter once again. You will notice that if the two metals are different, they will continue to generate electromotive force between them.
Explanation
The lemon contains citric acid (an organic acid), which acts as an electrolytic solution. Moreover, when metal is inserted into the lemon, a chemical reaction occurs between the acid and the metal, either with one of the metals or both. This can cause the metal wires to become charged (with excess electrons), resulting in one wire being more negative than the other. When connecting the two terminals with a conductor, the current is formed by the movement of negative charges from the negative terminal to the positive terminal.
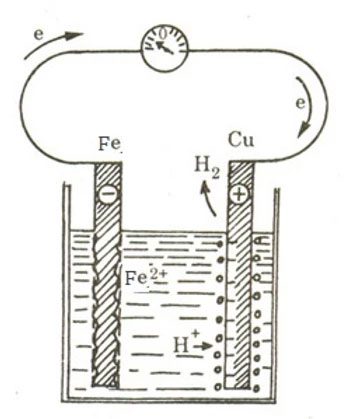
When two different metal rods (for example, copper and iron) are dipped into an electrolytic solution (containing ions), and a reaction occurs at the iron rod, voltage can appear.
Thus, we can conclude that to have a battery (electrochemical cell), two components are needed: an electrolytic solution and two metals of different nature. Based on this, we can infer that it is possible to replace the acid solution with a salt solution, although the generated voltage may be smaller.
Can the electricity generated from these lemons be used?
The answer is yes. By connecting batteries in series (the negative terminal of one battery connects to the positive terminal of another) or in parallel (the negative terminals are connected together, and the positive terminals are connected together), we can create a strong enough power source to light a small LED bulb.


















































