Lithium-ion batteries (Li-ion batteries) are widely used in devices such as mobile phones, laptops, and electric vehicles. Unlike nickel batteries, Li-ion batteries are more expensive but offer three times the charge-discharge cycles when used in electric vehicles. They also have a longer lifespan, are lighter, smaller, and can be charged much faster. Due to their unique chemical properties, Li-ion batteries have a specific charging process that differs from other types of batteries such as nickel or lead-acid.
Overview of the Lithium-ion Battery Charging Process
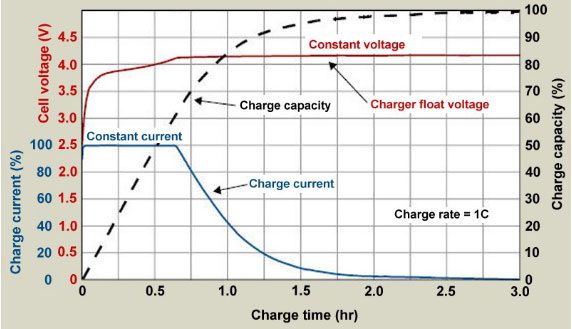
Constant Current Charging: During constant current charging, the current is kept stable, typically at C/2-C (where C is the battery capacity in Ah). The larger the charging current, the shorter the constant current charging process, but the constant voltage charging phase will take longer; however, the total charging time for both stages usually does not exceed 3 hours. Additionally, a high current will increase the battery’s temperature. It is essential to monitor the temperature closely during charging, as excessive heat can cause the battery to catch fire or explode.
Typically, the temperature should not exceed 45°C. Some Li-ion batteries that utilize Lithium Iron Phosphate (LiFePO4) technology can tolerate charging temperatures of up to 60°C. When using quick charge systems, they apply a steady current to the battery (constant current charging), meaning that a higher temperature limit corresponds to a larger charging current or a shorter charging time.
Li-ion battery charging process.
Constant Voltage Charging: In constant voltage charging mode, the charging voltage is generally maintained at 4.2V per cell. As the battery capacity gradually recovers, its electromotive force increases, causing the current to decrease. When the current drops below 3% C, the constant voltage charging phase concludes, at which point the battery capacity reaches about 99%. During constant current charging, the voltage across the battery terminals gradually increases. When the voltage reaches the electromotive force of a fully charged battery, the charger terminates the constant current charging and switches to constant voltage charging mode. The entire constant current charging duration usually lasts a maximum of about 1 hour (depending on the initial remaining capacity of the battery). At the end of the constant current charging process, the battery capacity has typically recovered about 70%.
In many cases (quick charge), the battery can be used immediately after charging (a method known as “charge-and-run”). While this reduces charging time and simplifies charger design, it can also decrease the battery’s lifespan. To ensure the battery’s longevity according to the manufacturer’s specifications, both constant voltage and constant current charging phases are typically required, which often takes considerably longer than the constant current phase alone.
Unlike nickel or lead-acid batteries, Li-ion batteries do not need and must not maintain charging voltage after the battery is full (when the charging current drops below 3% C) because the nature of Lithium-ion does not allow for overcharging; attempting to overcharge can cause the battery to heat up and potentially explode. Additionally, experts recommend not charging Li-ion batteries beyond 100% capacity as this reduces the battery lifespan. This issue will be clarified in the following section.
If the battery is fully charged, after charging stops, the open-circuit voltage of the battery will gradually decrease to a stable level of about 3.6 – 3.9V per cell. In contrast, if only constant current charging is applied, after charging stops, the battery voltage will drop further to around 3.3 – 3.5V.
Since Lithium-ion batteries also exhibit self-discharge when not in use, in some cases, to fully charge the battery, in addition to using constant current and constant voltage processes, a short pulse charging technique is often combined.
For instance, when the battery voltage reaches 4.2V per cell, the charging process will stop immediately. At this point, the battery voltage will gradually decrease; when the battery voltage drops to 4.05V per cell, the charging system resumes applying 4.2V per cell to continue the charging process.
This on-and-off action will occur continuously. As a result, the battery voltage will be kept stable in the range of 4.05 – 4.2V per cell, allowing the battery to charge deeper, preventing over-charging and extending the battery’s lifespan.
Over-charging Issues of Lithium-ion Batteries
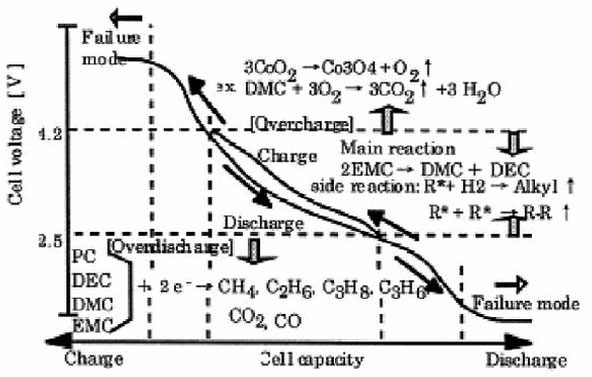
Typically, Li-ion batteries should only operate (charge/discharge) within the designed voltage range (below 4.2V per cell). However, in some cases, if the battery is fully charged and continues to receive current, the battery voltage will rise above 4.3V. At this point, the battery is considered to be overcharged.
When the battery voltage falls outside the safe operating range (above 4.2V per cell or below 2.5V per cell), its operation becomes unstable. Lithium metallic layers will form on the positive electrode, while the negative electrode will be strongly oxidized, reducing stability and generating CO2 gas within the battery, leading to increased pressure inside the battery. For safety, the charger must stop charging when the pressure in the cell reaches 200 psi.
If the charger lacks pressure monitoring and protection functions, the continuous generation of CO2 gas will cause the battery pressure to keep rising, while the battery temperature will also increase rapidly. When the pressure reaches about 500 psi, the battery temperature will be around 130-150 degrees Celsius, causing the safety membrane separating the cells to rupture, leading to potential combustion or even explosion.
Therefore, during the charging process, it is crucial to adhere to the temperature and voltage requirements for the cells.
Operation of the battery.
Li-ion batteries should generally not be over-discharged. When the battery voltage drops below 3.0V per cell, it is best to disconnect the battery from the circuit. If the voltage drops below 2.7V per cell, the battery’s internal protection circuit will automatically switch the battery to sleep mode. At this point, the battery cannot be recharged in the usual manner and requires a four-stage charging cycle as shown in the diagram.
In the four-stage charging cycle, in addition to the two stages of constant current and constant voltage similar to the normal Li-ion battery charging process, two stages of Pre-charge and Activation are added to restore the battery’s functionality.

Samsung lithium-ion battery cell.
Initially, during the Pre-charge phase, a small current (5-15% C) will be applied to the battery, and then the battery voltage will be monitored. If, after a predetermined period (testing time), the battery voltage does not increase or increases too slowly, the battery is considered irrecoverable. Conversely, if the voltage rises above 2.8V, the battery is still functional and can continue to charge. At this point, the charger switches to Activation mode to reactivate the battery’s operation.
In Activation mode, the current of 5-15% C continues until the battery voltage rises above 3V. The charger then resumes normal constant current and constant voltage charging.
When manufacturers sell batteries, they usually charge them to 40% capacity. However, over time, due to self-discharge, the battery capacity gradually decreases, which means the battery voltage drops. To avoid over-discharge, the battery should be periodically maintained by recharging it after being unused for an extended period.
Each Li-ion battery cell typically has an open-circuit voltage of about 3.5V. In systems like electric vehicles, to supply power to the main propulsion motor and other electrical devices in the vehicle, cells are often connected in series-parallel until reaching a DC bus voltage of about 200VDC or higher.
Cell Balancing Issues
Factors such as manufacturing tolerances for the cells; varying temperatures affecting each cell during operation; or the effects of aging can cause the properties of the cells to differ. Some cells may have slightly higher voltages, while others may have slightly lower voltages compared to the others, meaning the cells are unbalanced.
During charging, cells with higher voltages will fill up first while some other cells remain uncharged. If charging continues, those cells will overcharge, causing temperature and pressure to rise (as analyzed above), reducing the overall battery lifespan and potentially damaging that cell. Conversely, during discharging, cells with lower voltages will deplete faster. If deep discharging continues, those cells will over-discharge, further shortening battery lifespan. When a cell becomes damaged, usually the entire battery system must be replaced; if only the damaged cell is replaced (which may be possible in some cases), that new cell will still have different properties compared to the remaining cells, meaning the risk of imbalance will still exist.
The more cells connected in series, the higher the risk of imbalance and the lower the reliability. Studies have shown that if a battery system consists of n cells, the probability of imbalance increases n-fold compared to just one cell operating independently.
To mitigate this issue, several approaches can be considered. First, efforts will be made to select cells with relatively consistent specifications for pairing. The cells will then be connected in series-parallel rather than just in series, as this allows for a circulating current to help balance the cells (self-balancing). Furthermore, during usage, temperature must be closely monitored to ensure even distribution across the cells.
However, to thoroughly address the issue of battery imbalance in Li-ion batteries used in electric vehicles, the Battery Management System (BMS) must closely monitor the capacity of each cell (State of Charge – SOC). If any imbalance is detected, the BMS needs to take specific actions to bring the cells back into balance. There are two methods to achieve this: active balancing and passive balancing.
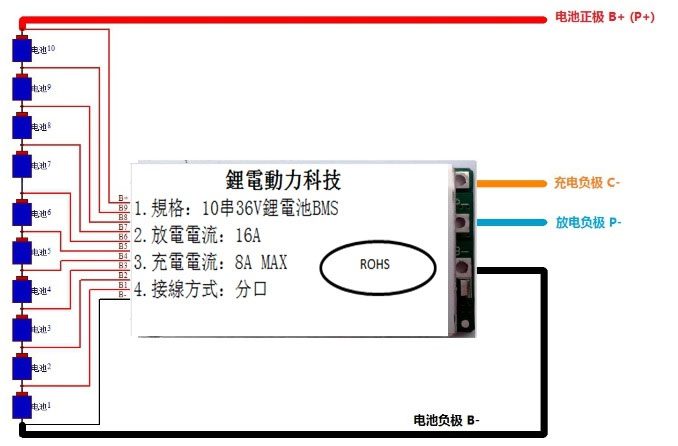
36V balancing circuit for electric bicycles.
The active balancing method transfers excess energy from cells with higher capacity to those with lower capacity. This method has the advantage of maintaining voltage balance across the system without energy loss, as energy is exchanged among the cells. However, designing an independent charging source for each cell is impractical. Voltage balancing is performed sequentially for one or a group of cells, thus requiring a significant amount of time to fully charge the entire battery pack.
The passive balancing method is simpler than active balancing but results in energy loss due to resistance. The charger needs to stop charging immediately when any one cell is fully charged. Subsequently, the fully charged cell will discharge through a resistor until its voltage matches that of the lower-capacity cell. After that, the charger can resume operation, and the cycle repeats until all cells are fully charged.
Thus, during the charging process, besides adhering to proper charging procedures, the charger must closely coordinate with the BMS to implement cell balancing techniques to fully charge the cells and prevent imbalances between them, thereby extending the lifespan of the entire battery pack.
The Effect of Temperature on the Lithium-ion Charging Process
As mentioned in the previous section, the charging and discharging activities of batteries are significantly affected by temperature. In general, all types of batteries can operate within a fairly broad temperature range. For Li-ion batteries, this range is from 0°C to 45°C during charging and from 0°C to 60°C during discharging. Some newer lithium-based batteries, such as Lithium Iron Phosphate (LiFePO4) or Lithium Polymer, allow for a slightly wider operating temperature range. Within this range, battery characteristics are relatively stable, and energy efficiency is high. However, outside of this temperature range, whether at extremely low or high temperatures, battery performance is severely impacted, and the chemical reactions within the battery slow down, which means the current generated or absorbed by the battery will decrease compared to when operating within the optimal range.
For Li-ion batteries in general, it has been demonstrated that the optimal operating temperature range is from 5°C to 45°C. Below 5°C, charging current should be reduced, and when the temperature drops below 0°C (freezing point), charging should be halted immediately.
Conversely, at temperatures above 45°C, the performance of the battery becomes more vigorous, meaning it can discharge or charge at a current greater than its nominal current (C). However, in both cases (both excessively low and high temperatures), the internal resistance of the battery increases, which can reduce battery lifespan if charging is attempted.
Requirements for Using Li-ion Batteries
- Turn off all devices powered by the battery that needs charging. This way, the current and voltage measurements will provide accurate results, reflecting the correct charging process parameters.
- Avoid charging when ambient temperatures are too low or too high.
- Stop charging immediately if the battery temperature rises abnormally.
- Stop charging when the battery capacity reaches around 90% to 99%. This is better for the battery than charging it to 100% or more. Typically, chargers have capacity indicators and will automatically cut off when the capacity reaches 90% to 99%. If not, users must monitor and cut off the charging. This will increase the battery’s lifespan.
- Before storing a battery that will not be used for an extended period, it should be charged to around 40-50% capacity to avoid the risk of over-discharge due to self-discharge.
- Avoid attempting to charge a battery with an electromotive force below 2.7V/cell (which has been over-discharged) with standard chargers (which only support constant current and constant voltage modes); instead, use specialized chargers that support all four modes: Pre-charge, Activation, Constant Current, Constant Voltage.

















































