On the morning of February 28, at Bach Mai Hospital in Hanoi, Vietnamese experts held a video conference discussing the diagnosis and treatment of H5N1 influenza with scientists from Japan and Australia. This is the first remote seminar on A influenza to be organized.
During the seminar, Dr. Nguyen Thi Tuong Van, Deputy Head of the Emergency Department at the Institute of Tropical Medicine, presented her experience in treating H5N1 patients, highlighting the case of Nguyen Sy Tuan from Thai Binh, who was saved after nearly three months in the hospital with multiple severe complications. This patient has since recovered his health and weight nearly back to normal.
Dr. Tuong Van stated that A influenza remains a global concern as outbreaks in poultry continue to occur in various regions. Therefore, countries are eager to learn about Vietnam’s treatment experience, where the highest number of patients has been recorded, and many have been successfully treated.
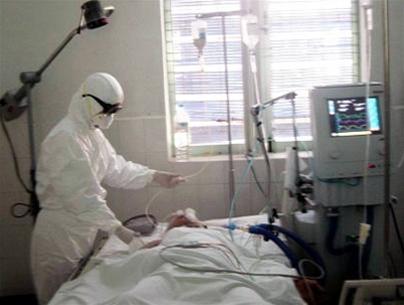
A patient suffering from acute respiratory infection caused by H5N1 influenza
being treated at the Tropical Diseases Hospital in early 2005 (Photo: TTO)
“This morning’s discussion highlighted a significant difference in treatment perspectives for A influenza between Vietnam and the rest of the world,” Dr. Van remarked. According to international experts, the most crucial factor in treating H5N1 patients is the use of Tamiflu to inhibit virus development, while the use of high-dose antibiotics and corticosteroids is not highly regarded.
However, practical experience in Vietnam shows that in cases of A influenza, as well as previously with SARS, the cause of mortality is not the virus itself but complications such as secondary infections, multi-organ failure, and pulmonary edema. Therefore, to save patients, a comprehensive treatment regimen is necessary, including antiviral medications, antibiotics, anti-inflammatory drugs, and other therapies tailored to specific circumstances. With this approach, the Institute of Tropical Medicine has successfully treated numerous patients during outbreaks of SARS and A influenza.
During the remote seminar, Japanese experts also presented images of lung damage in H5N1 patients to discuss diagnostic procedures. Meanwhile, Australian representatives (who have not yet encountered any patients) shared their plans for pandemic influenza prevention.
Dr. Tuong Van indicated that in the future, international exchanges via video conferencing on the diagnosis and treatment of respiratory diseases will be held more frequently.
|
Professor Nguyen Thu Van, Director of the Vaccine and Biological Products Company No. 1 at the Central Hygiene and Epidemiology Institute, reported that with the current progress, the research and production of H5N1 influenza vaccine at this facility is expected to be completed by the end of 2006. In the meantime, the institute can produce 2-3 million doses per year, sufficient for approximately 1 million people. Currently, Vietnam’s H5N1 vaccine has passed pre-clinical trials with positive results. This formulation will undergo clinical trials (on humans) once it is assessed by the World Health Organization as meeting the necessary conditions. The Central Hygiene and Epidemiology Institute has completed the installation of the production line for this vaccine and is ready to operate as soon as permission is granted. |