Under certain conditions, lithium-ion batteries can enter a state of continuous overheating, which is uncontrollable and can lead to fire and explosion.
According to the Lawrence Berkeley National Laboratory at the University of California, USA, the movement of electrons and lithium ions is the main factor that generates electricity in lithium-ion batteries. However, the charging and discharging processes of the battery typically produce a small amount of heat. In ideal conditions, heat can dissipate from the battery.

Lithium-ion batteries can experience thermal runaway, causing the battery to overheat, catch fire, and explode (Illustration).
In certain cases, lithium-ion batteries can generate heat at a much higher rate than they can dissipate it. This causes the battery to overheat and can lead to a chain reaction known as thermal runaway.
When this phenomenon occurs, the lithium-ion cells inside the battery enter a state of continuous overheating that is uncontrollable. This is the primary cause of the battery overheating, leading to fire and explosion.
Furthermore, researchers at Berkeley Laboratory have pointed out that the presence of localized current within the battery while at rest after fast charging can also be one of the causes behind thermal runaway.
Moreover, the design of the battery itself plays a crucial role in ensuring safety against potential fire and explosion incidents.
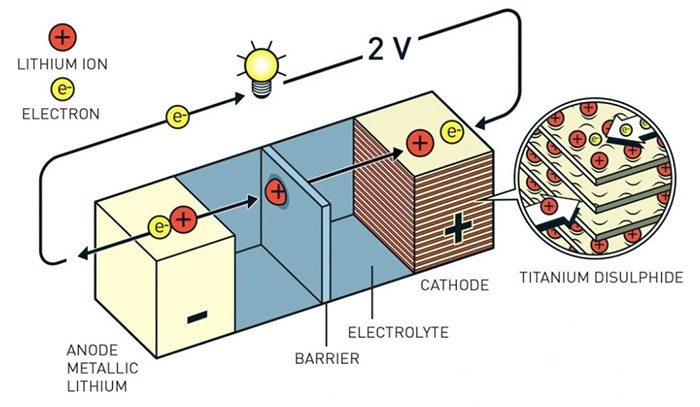
Structure of a lithium-ion battery (Image: Science Daily).
Typically, to fit into a compact space, the main components of the battery are designed to be as small and lightweight as possible. However, this also means that the battery components are often made with thin, fragile walls that are poorly resistant to impact.
If the battery is punctured or deformed due to physical impact, electrical sparks can occur and cause a fire. This situation can be commonly found in poorly manufactured batteries, which are incomplete and have unclear origins.
In an interview, Professor Stanley Whittingham, known as the father of the lithium-ion battery, specifically warned users about the dangers of using low-quality batteries.
He stated that safety issues with lithium-ion batteries stem from the fact that many types of batteries are produced at low costs, resulting in lower safety standards, which can lead to fire and explosion during use.
Professor Whittingham emphasized that to ensure safety, we must ensure that our batteries meet safety certifications by purchasing them from reputable sources.
Additionally, experts advise that for businesses handling a large number of products containing lithium-ion batteries, it is crucial to understand the risks and how to prevent fire outbreaks.
One of the key measures is to ensure that the storage location for batteries is not exposed to direct sunlight, located near large heat sources, and kept away from flammable materials such as blankets, pillows, sheets, and mattresses…


















































