All the water on our planet, including rainwater, contains chemical compounds that scientists refer to as “salts.” However, not all types of water taste salty. We all know that seawater always tastes salty, while rainwater, pond water, and river water do not. So have you ever wondered why there is such a difference?
How Salty is Seawater?
Let’s conduct a small experiment to see how salty seawater is. Take a glass of plain drinking water, which you can obviously enjoy drinking. Now add a bit of salt; you start to taste the saltiness but can still drink it as the level of saltiness hasn’t reached your tolerance yet. Now keep adding salt, and soon enough, you’ll find that the water is undrinkable.
Your taste buds will vehemently protest that this water is too salty to drink. Seawater is similar; humans cannot drink it. Seawater is completely different from the freshwater we consume daily. It contains a vast amount of dissolved compounds that the human body cannot accept.

Seawater is completely different from the freshwater we consume daily.
So, how salty is seawater? Some chemists estimate that the oceans on Earth contain over 50 million trillion tons of dissolved substances. If the salt in seawater were extracted and laid out on land, it would create a layer over 152 meters thick spread across the continents. This height is equivalent to a modern 40-story building.
Let’s compare the salt content of seawater to that of freshwater in ponds. In 28 liters of seawater, there is about 1 kg of salt. In contrast, water in a typical pond contains only about 4.54 grams of various salts. Therefore, we can conclude that seawater is approximately 220 times saltier than freshwater in ponds.
This has sparked curiosity among scientists: Why does freshwater from rivers taste salty when it flows into the ocean? Where do the origins of the sea and the “salts” it contains come from? How can we explain the origins of the vast chemical components in seawater? All these questions and more are what scientists seek answers to.
The Origin of the Oceans
The oceans (or seas) on our planet include: the North and South Pacific, the North and South Atlantic, the Indian Ocean, the Arctic Ocean, and the Southern Ocean. Based on ancient fossils found on the ocean floor, scientists estimate that the oceans are over 500 million years old. To date, there are many theories explaining the origin of the oceans. However, no single theory has been able to explain all aspects of the issue.
Many studies about Earth agree with the theory that both the atmosphere and the oceans accumulated gradually from the time of geological formation through the “outgassing” process of the Earth. According to this theory, the oceans originated from water vapor and other gases released from the Earth’s molten magma, which then rose high and cooled to form clouds above.

The ocean is over 500 million years old.
After the Earth’s surface cooled below the boiling point of water, rain began to fall and continued for many centuries. Once all the water fell, it covered almost the entire surface of the Earth, giving birth to the first primordial oceans. At the same time, gravity kept the water trapped so it wouldn’t escape Earth.
The Origin of Salt
Seawater is a complex mixture of various mineral salts and compounds from the remains of decomposed marine organisms. Most of the mineral salts in the ocean accumulate gradually. This is the result of the cooling processes of magma on the Earth’s crust due to weathering and erosion. When mountains formed, rainwater and streams carried minerals from the land to the sea, gradually accumulating them to the levels we see today.
Some salts in the ocean also originate from rocks and sediments beneath the ocean floor. Another source of seawater’s salt comes from solids and gases released from the Earth’s crust through volcanic vents. Volcanoes bring compounds from within the Earth’s core to the surface, accumulating in the ocean.
If Freshwater Flows into the Ocean, Why is Seawater Still Salty?
Freshwater from rivers like the Amazon, Mississippi, and Mekong continuously flows into the Pacific, Atlantic, and other oceans, yet all seawater remains salty. Why doesn’t the freshwater dilute the seawater? The reason the saltiness of the ocean is the result of many natural processes, and the salt content in the ocean is just one of the factors contributing to this salinity.
Initially, the ancient sea contained only a small amount of salt and did not achieve the salinity it has today. However, after the first rains fell on the young Earth hundreds of millions of years ago, the flowing water broke down geological layers and transported minerals into the ocean. Since then, the oceans have gradually become saltier. It is estimated that rivers in the United States contribute 225 million tons of dissolved solids and 523 million tons of sediments to the ocean every year.
Recent calculations have shown that the amount of dissolved solids from land ranges from about 2.3 tons per square kilometer in Australia to 46.3 tons per square kilometer in Europe. It is estimated that all the rivers in the world carry approximately 4 billion tons of dissolved mineral salts to the ocean each year. This salt settles at the ocean floor, gradually forming new sediment layers. In other words, the amount of salt entering and exiting all the oceans on Earth is always balanced.
Thus, the amount of salt entering the ocean in dissolved form and leaving the ocean in sediment form still does not explain the source of the seawater’s saltiness. We know that salt always concentrates in the sea and cannot move with water vapor. When the sun heats the ocean surface, almost pure water vapor rises, but the mineral salts remain in the sea. This process is part of a continuous cycle occurring between the Earth and the atmosphere: The Water Cycle.
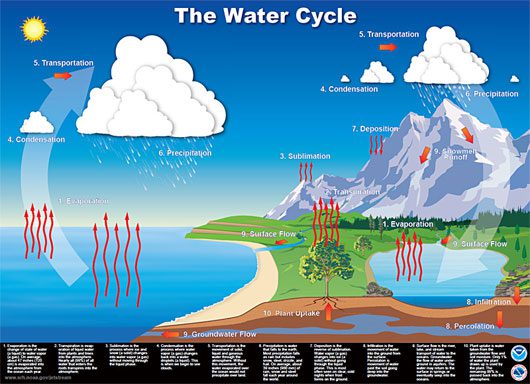
The Water Cycle.
Water vapor rises from the ocean surface and is carried elsewhere by winds. When the water vapor meets cooler air aloft, it condenses (changing from gas to liquid) and falls to the ground as rain. Rain on land is collected by rivers and streams, eventually flowing back into the ocean. And this cycle continues perpetually. This is why the water in rivers on land does not taste salty, but when it flows into the ocean, it continues to dissolve the salt that remains in the sea, retaining its salty taste. In fact, since the first rains fell, the seas have gradually become saltier.
Seawater is Not Simple
Scientists have studied seawater for centuries. However, they have yet to fully understand its chemical components. One reason for this is the lack of proper methods and processes to measure the components in seawater. The underlying reason hindering the research process for scientists is the vast size of the Oceans, which cover about 70% of the Earth’s surface, and the highly complex system of chemical compounds inherent in the marine environment, among which some elements continually change over time.
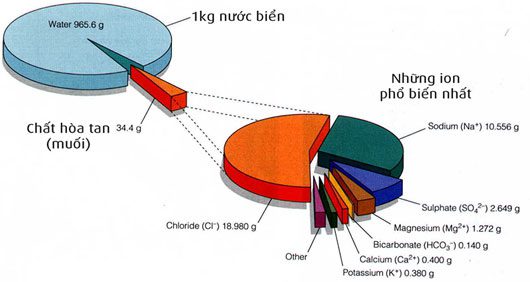
Composition of seawater.
As of now, only 72 chemical elements have been discovered in seawater. This number is quite small compared to the actual number of compounds that exist in the ocean. Some scientists believe that all naturally occurring chemical elements on Earth are present in seawater. Furthermore, these elements combine in various ways, both in dissolved forms and as precipitates that settle to the ocean floor, forming sediments. However, even after precipitation, these compounds can still change their chemical composition due to the ongoing processes occurring in the marine environment.
Variability of Seawater Salinity
Oceanographers use parts per thousand (o/oo) to measure salinity (considering all types of salts) and the concentration of certain specific components in seawater such as NaCl, sodium, magnesium, etc. Thus, when we refer to a salinity of 35 o/oo, it means there are 35 pounds (15 kg) of salt in 1000 pounds of seawater. Similarly, a NaCl concentration of 10 o/oo indicates there are 10 pounds of NaCl in 1000 pounds of seawater.
Seawater salinity also varies. It depends on factors such as the degree of ice melting, the amount of freshwater flowing from rivers, evaporation rates, rainfall, snowfall, winds, wave movements, and ocean currents. All these factors contribute to the differences in seawater salinity across various regions of the world.
The Saltiest Seawater…
The saltiest body of water (40 o/oo) belongs to the Red Sea and the Persian Gulf. These are the two areas with the highest evaporation rates of seawater. Comparing major oceans, the Atlantic Ocean has the highest salinity, with an average salinity of about 37.9 o/oo. Within the North Atlantic, the Sargasso Sea has the highest salinity, covering an area of approximately 5.18 km2. The relatively high salinity in this area is partly due to temperature. The water temperature here is quite high (around 28oC), leading to high evaporation rates. Additionally, this marine area is located quite far from the mainland (about 2000 km west of the Canary Islands), thus it does not receive freshwater from rivers.
Black Sea – The saltiest seawater in the world.
The areas with the lowest seawater salinity belong to the Arctic and Antarctic Oceans. This is due to the low temperatures in these regions, and moreover, seawater is continuously diluted by melting ice and persistent rainfall. Coastal bays also tend to have lower salinity than average. For example, the Baltic Sea has a salinity ranging from 5 to 15 o/oo, and the Black Sea has a salinity of less than 20 o/oo. Most of these marine areas receive several billion tons of freshwater daily.
Similarly, the salinity of coastal waters varies according to the time of year and geographical location. For example, the coastal area in Miami, USA, has seawater salinity that changes from 34.8 o/oo in October to 36.4 o/oo in May and June. In contrast, during the same periods, the coastline of Astoria, Oregon, has seawater salinity of 0.3 o/oo in April and May, rising to 2.6 o/oo in October.
The difference is due to the fact that the Miami coastal area is less diluted by freshwater than the marine area of Astoria. The waters around Astoria, on the other hand, are diluted by freshwater from the Columbia River.
In general, the salt composition in seawater comes from both pre-existing sources and various sources on land. This results in seawater salinity typically ranging from 22 o/oo to below 38 o/oo. On a global scale, the average salinity of seawater is about 35 o/oo. This average salinity was estimated by scientist William Dittmar in 1884 based on the analysis of 77 seawater samples collected from various locations around the world during a scientific expedition conducted by Britain.
The scientific expedition was initiated by the British government at the proposal of the Royal Society to study marine organisms, examine the chemical and physical properties of the sea, survey chemical substances on the ocean floor, and monitor seawater temperature. The journey began in 1872 and concluded after four years of sailing, covering a distance of 68,890 nautical miles. To date, this remains the longest marine expedition recorded.
The 77 seawater samples collected by Dittmar were analyzed for their chemical composition (main components) and documented. These samples are still regarded as the most comprehensively collected seawater samples to date. Recent studies have analyzed and resampled with the aid of technological advancements. The results obtained from these experiments indicate that Dittmar’s notes retain a high degree of accuracy. The component results of the 77 seawater samples are recorded in the table below:
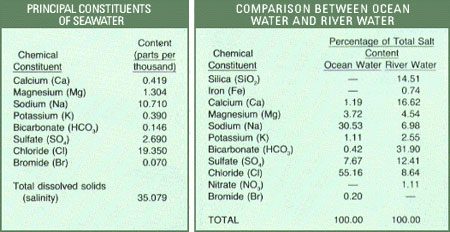
Composition of 77 seawater samples.
Differences Between River Water and Seawater
From the analysis table, it is evident that seawater consists of a variety of different compounds. Sodium and chlorine (combined as NaCl, commonly known as table salt) account for 85% of the dissolved substances in seawater. This is the primary factor contributing to the salty taste of seawater. In comparison to river water, we find that rivers supply more calcium than chlorine to the ocean. However, the oceans still contain 46 times more chlorine than calcium.
Additionally, river water contains silicate and iron compounds, which are absent in seawater. Calcium bicarbonate constitutes nearly 50% of the dissolved solids in river water but accounts for less than 2% in seawater.
How Do Marine Organisms Affect Seawater Composition?
By comparing the differences between river water and seawater, we can somewhat explain the influence of marine organisms on seawater composition. As we know, seawater is not just a simple salt solution but also contains many other substances derived from marine life. Marine organisms also utilize these substances in seawater for their survival. Various shellfish (oysters, clams, snails, etc.) can extract calcium from seawater to form shells and skeletons. Similarly, many types of plankton and crustaceans use calcium from the sea to form their skeletons.

Many types of plankton and crustaceans use calcium from the sea to form their skeletons.
At the same time, plankton also affects seawater composition through the waste they produce. Moreover, some animals can continuously secrete substances they produce to avoid detection by predators. Lobsters can combine copper and cobalt, while some types of snails can secrete lead. Sponges, on the other hand, can extract vanadium and also separate iodine from seawater.
Therefore, marine organisms also significantly influence the composition of seawater. However, there are certain chemical elements in seawater that no organism can break down. Notably, to this day, no species has been found that can eliminate sodium from seawater.
The Ratios of Major Seawater Components Are Relatively Constant
Generally, the ratios of major components in seawater worldwide remain relatively unchanged. Dittmar’s 77 seawater samples show virtually no distinct differences in their relative composition and density across various locations around the globe. Dittmar conducted his analysis over nine continuous years and concluded that NaCl, magnesium, sulfate, calcium, and potassium account for up to 99% of the dissolved solids in seawater.
In other words, the results indicate that while salinity and the total amount of salts contained in seawater may vary across different regions of the world, the ratios of major components (such as NaCl as an example) in the total compounds remain nearly constant. However, the ratios of less common elements such as aluminum, copper, tin, and gases like oxygen, CO2, and nitrogen do vary between different marine waters. Nevertheless, because the major components of seawater exhibit minimal differences, scientists can use this information to assess the overall impact of factors such as temperature and pressure on seawater salinity.
Conclusion
The salinity of seawater originates from the gradual accumulation of compounds eroded from the Earth’s crust that flow into the oceans. Solid materials and gases released from volcanic eruptions on land are also carried to the ocean by the wind. Compounds released from sediment layers on the ocean floor contribute to the current salinity of seawater.

The salinity of seawater can increase or decrease depending on sea surface temperature.
The salinity of seawater can increase or decrease based on sea surface temperature, rainfall, and the geographical location of the sea, particularly whether it receives abundant freshwater. The average salinity of seawater is 35 o/oo, with the highest salinity found in the Red Sea and the Persian Gulf, reaching a record 40 o/oo. The areas with the lowest salinity are typically found in polar regions, coastal waters, or near the mouths of major rivers.
Seawater is not only saltier than river water, but the composition and ratios of the dissolved salts within it also differ. NaCl (table salt) accounts for 85% of the dissolved solids in seawater. This is the primary reason for the characteristic salinity of seawater.
This summarizes the reasons why seawater has its current salty taste. By seeking answers to this question, we have had the opportunity to review the history of ocean formation on Earth, the chemical components of seawater, and the impact of marine organisms on the marine environment.




















































