The periodic table is not just a simple list; it is an encyclopedia that reveals the mysterious relationships between elements. From the few basic elements discovered by Humphrey Davy in the early 19th century to the 118 elements we know today, each addition is not just a numerical increase but a leap in human understanding of the natural world.
Each cell of the periodic table represents a profound discovery about nature. It continues to expand, like the boundaries of science being pushed forward. Every time scientists discover a new element, it is akin to adding a bright color to the vast painting of the universe.
The discovery of new elements often accompanies the birth of new technologies, the development of new materials, and even the starting point of a new industrial revolution. As science progresses, people begin to wonder: Has the periodic table of chemical elements reached its conclusion?
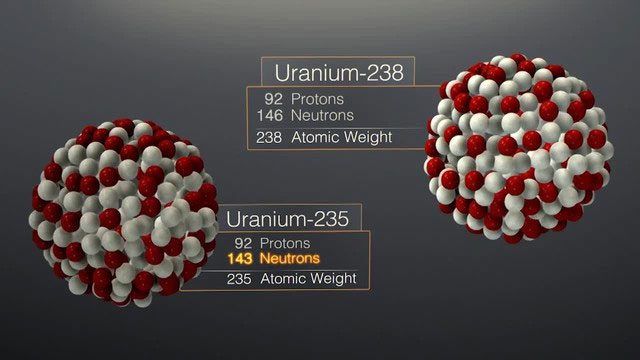
Uranium element.
The Origins of the Periodic Table
In 1869, when Russian chemist Dmitri Ivanovich Mendeleev was classifying chemical elements, he discovered a periodic pattern between the atomic mass and chemical properties of the elements.
He arranged the elements according to their atomic masses and predicted the existence and properties of several unknown elements. This discovery not only filled gaps in the periodic table of that time but also foreshadowed the discovery of new elements in the future.
Mendeleev’s original periodic table contained only the elements known at that time, and over time, more elements were discovered, expanding the table. Whenever new elements were discovered, the periodic table would be confirmed and recognized further.
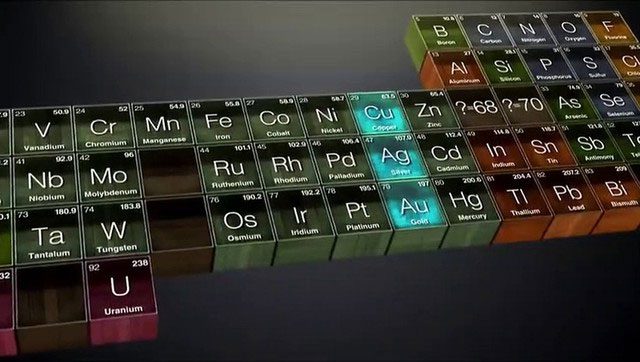
Each cell of the periodic table represents a profound discovery about nature.
It is worth noting that Mendeleev’s work was not an isolated achievement. Other scientists, such as Julius Lothar von Meyer, independently proposed similar classification systems for the elements. Over time, the periodic table has become the foundation of modern chemistry.
With the rapid development of physics in the early 20th century, scientists gradually revealed the complexity of the internal structure of atoms. This discovery triggered a reevaluation of the periodic table of elements. Atomic numbers gradually replaced atomic mass as the main basis for arranging the elements. The new structure of the periodic table is more accurate and orderly, providing a more precise description of the relationships between elements.
Today, the periodic table is not only an essential tool for chemistry students but also a guide for scientists exploring the material world.

Superheavy elements have atomic numbers greater than 92 (uranium).
Exploring Superheavy Elements
In the history of exploring the periodic table of elements, synthesizing superheavy elements has been a challenging and innovative field. Superheavy elements are those with atomic numbers greater than 92 (uranium); they do not exist in nature and can only be obtained artificially.
The process of synthesizing superheavy elements is typically carried out in particle accelerators. Scientists accelerate lighter nuclei to extremely high speeds, causing them to collide with target nuclei to create new elements through nuclear reactions.
However, synthesizing superheavy elements is not an easy task. Typically, the half-lives of synthesized superheavy elements are very short, with some existing for only a few milliseconds. This makes studying their chemical properties very difficult. Scientists must conduct experiments and measurements quickly and within extremely short time frames.
Currently, the heaviest element is Oganesson (atomic number 118). (Illustration).
Although the stability of superheavy elements presents a significant challenge, theoretical physicists still predict the existence of a “stable zone.” In this zone, certain combinations of atomic numbers and neutron numbers may produce relatively stable superheavy elements. This theory has inspired scientists to explore new elements.
Currently, scientists have synthesized several superheavy elements, the heaviest of which is Oganesson (atomic number 118). However, the “expedition” does not stop there; scientists are searching for heavier elements and those that may exist in stable regions.
The exploration of superheavy elements is crucial not only for understanding the structure and nuclear forces of atomic nuclei but also for the potential development of new materials and technologies. Although the practical applications of these elements remain unexplored, their synthesis challenges and expands the limits of human knowledge.
Researching superheavy elements can help us understand processes inside stars better. (Illustration).
The Potential of New Elements
In the exploration of the periodic table, the potential for new elements is limitless. Scientists are optimistic that more elements can be synthesized and acknowledge the importance of this task.
With the continuous advancement of science and technology, especially particle accelerators and detection technology, we have reason to believe that the ability to synthesize new elements will greatly increase. The development of these technologies may lead to new breakthroughs, allowing us to explore previously unknown elements.
The discovery of new elements not only increases the quantity but, more importantly, they may possess unique physical and chemical properties. These properties could revolutionize materials science, energy development, and even medical research. Each newly discovered element has the potential to provide us with new clues about how the universe works.
For example, studying superheavy elements may help us understand better the processes inside stars and how elements are formed in the universe. While the prospects are exciting, scientists will face numerous challenges on the path to synthesizing new elements. One challenge is how to stabilize superheavy elements, as they often have very short lifespans.
Measuring the properties of these superheavy elements is very difficult. (Illustration).
Another challenge is how to measure the properties of these superheavy elements, as they are created and exist for a very limited time. At the same time, scientists also need to understand the behavior of these elements to clarify their nature and properties.
To overcome these challenges, scientists will continue to improve experimental techniques and theoretical models to better predict and synthesize new elements. They also need to continue advancing detection technology and particle accelerators to enhance the accuracy and efficiency of experiments.
This requires collaboration and effort from scientists worldwide to collectively promote the exploration of the periodic table of chemical elements. There is still no definitive answer to whether the periodic table has reached its conclusion. However, this unknown field has inspired countless scientists with curiosity and a desire to explore.