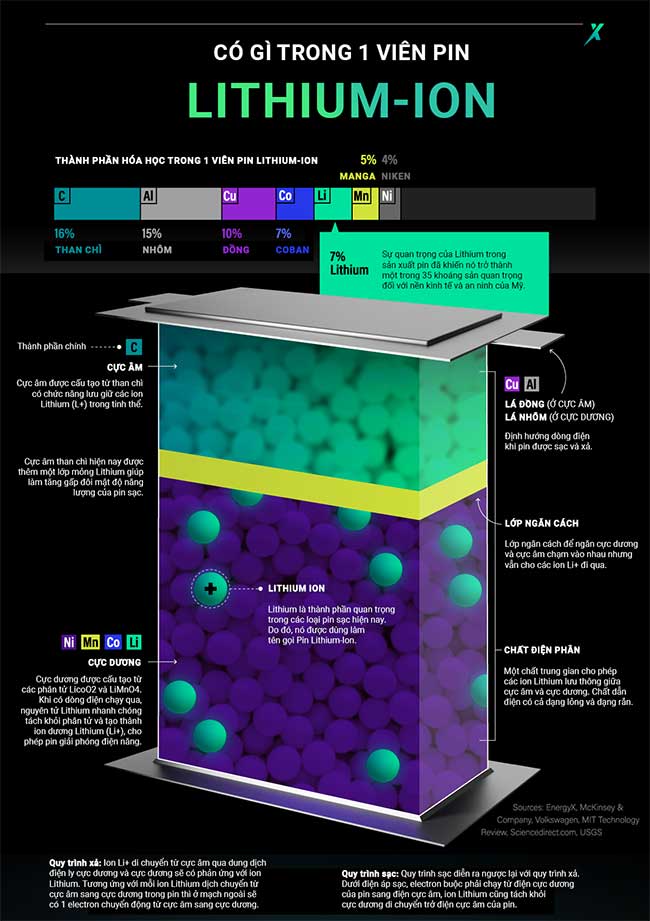
Winning the Nobel Prize in Chemistry in 2019, Lithium-ion batteries have become widely adopted and serve as the primary power source for most mobile devices today, from smartphones to electric vehicles.
During charging, Lithium ions move from the positive electrode to the negative electrode, and vice versa during discharging (the usage process).
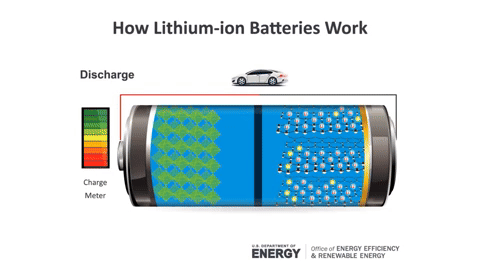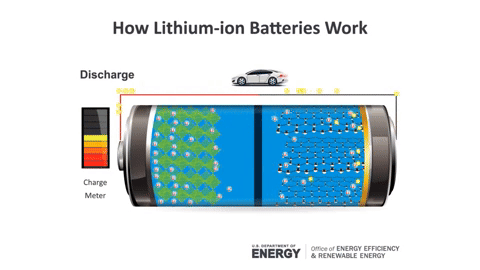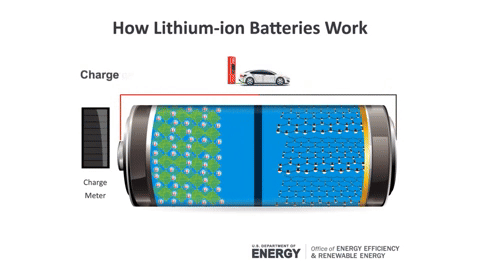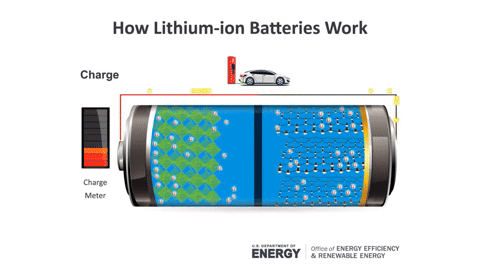
Lithium batteries are formed from four main components:
Negative Electrode: Composed of graphite (graphene) and other carbon materials that serve to store Lithium ions (Li+) within the crystal structure.
Positive Electrode: Made from LiCoO2 and LiMnO4. When an electric current flows, Lithium atoms quickly detach from the structure to form positive Lithium ions, Li+.
Electrolyte: This is a substance that conducts Lithium ions between the electrodes, consisting of salts, solvents, and additives.
Separator: Typically made from PE or PP plastic, it acts as a physical barrier to keep the positive and negative electrodes apart. However, Li+ ions can still pass through.