Technological advancement and sustainability are the foundations of our modern era. As more people are making conscious ecological choices, one such choice is electric transportation. Much has been discussed about electric vehicles, their environmental friendliness, and whether they can replace fossil fuel-powered vehicles. However, there is very little clear information regarding battery recycling.
Batteries, an essential component of all types of electric vehicles, are also experiencing rapid development. Like everything else, batteries have a lifecycle; so what happens to them when they are no longer useful?
Batteries are essential components of all types of electric vehicles.
To begin with, it is important to note that the type of battery we are discussing here is lithium-ion (Li-ion). They are widely used in most devices, on wireless gadgets, and even in electric cars. They have a high energy density and are rechargeable.
Over time, their ability to hold a charge and recharge diminishes, after which they can no longer be used safely and must be replaced. The battery pack in electric cars consists of multiple lithium-ion cells connected together and linked to a battery management system. This system regulates the charge and discharge rates of each cell in the battery pack, thereby maximizing their lifespan.

Lithium-ion batteries are ubiquitous in modern times.
How Are Used Batteries Recycled?
The first step in recycling lithium-ion batteries is to break them down into their constituent components. Understanding the structure of the battery will help us identify these components.
Structure of Lithium-ion Batteries
Lithium-ion batteries consist of a positive terminal, a negative terminal, a separator, and an electrolyte, all contained within a casing – lithium-ion batteries are made from various compounds. The positive terminal is typically made of graphite, while the negative terminal comprises various lithium oxides and phosphides. They also contain transition metals such as iron, nickel, manganese, and cobalt.
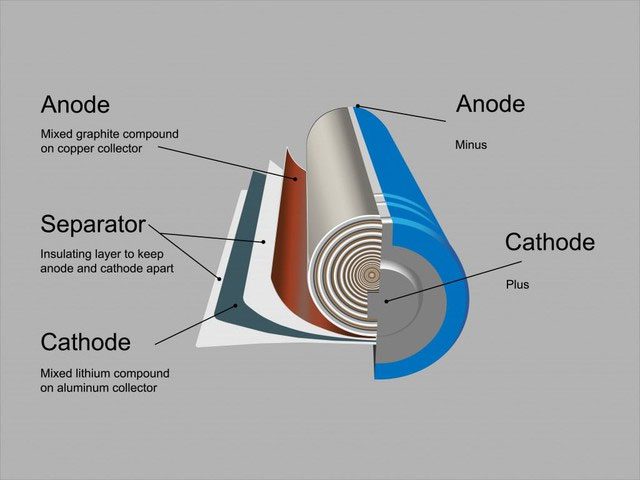
Structure of lithium-ion batteries.
The negative terminal is the most valuable part of the lithium-ion battery, as most negative terminals are made from cobalt. Consequently, recycling efforts revolve around recovering the negative terminal material and feeding it into various recycling processes. However, over time, recycling has shifted towards recovering as many materials as possible.
Different Battery Recycling Methods
Currently, battery recycling is carried out through three main methods: hydrometallurgical, pyrometallurgical, and direct physical recycling.
1. Hydrometallurgical Recycling
This recycling method primarily relies on filtering or processing with concentrated acids. Batteries are pre-processed to separate plastics and other materials used in the batteries. The used negative terminal material is treated with various acids to form a metal ion solution, from which metals can be easily separated. Commonly used acids include hydrochloric acid and sulfuric acid. Any residue generated from the reaction is reprocessed with acid; this method can extract up to 99% cobalt and lithium, as well as 98% copper.
In newer techniques, milder acids like oxalic and phosphoric acid are also used, yielding elements like manganese and nickel. This method is highly versatile and can be applied to both cobalt-type lithium-ion batteries and NCM (nickel-copper-manganese) batteries.

Apart from its industrial uses, cobalt is crucial for lithium-ion batteries and is the most sought-after metal in the recycling process.
2. Pyrometallurgical Recycling
The term “pyro” relates to high temperatures, and pyrometallurgical recycling is no different. Disassembled batteries are placed in a furnace, where they undergo a heating process. Gradually increasing temperatures lead to the evaporation of the electrolyte (preheating stage) and plastics (pyrolysis stage) present in the batteries.
The formation of metal alloys occurs at the highest temperatures in the furnace (melting stage). Important metals such as cobalt, copper, and nickel are recovered at this stage, while lithium is lost. To this day, this remains the most reliable technique for recovering cobalt from recycled batteries. However, this method is increasingly losing relevance because it does not recover lithium and is essentially useless for batteries that do not contain cobalt.

Compressing the powder at high temperatures to create a new negative terminal (sintering) is very useful in regenerating energy density and charge retention capabilities of the negative terminal material.
3. Direct Physical Recycling
In physical recycling, batteries are dismantled and processed using CO2 in a supercritical state (a state without a clear distinction between liquid and gas). This causes the separation of the electrolyte. While the electrolyte is recovered, the used cells are broken down into separate physical metals such as copper and aluminum.
The remaining material forms a large part of the negative terminal and is sintered at high temperatures to increase their energy density and electrochemical performance. Physical recycling is often reserved for lithium-ion batteries made from iron and manganese, which typically do not contain cobalt.
If we only rely on extracting raw materials, it is likely that the planet’s resources will deplete, similar to how we are treating fossil fuels. Battery recycling technology promises to recover most components of the battery, making it not only sustainable but also profitable as an industry!