Monash University (Australia) to Lead Research Program for Innovative Implantable Heart Devices, aiming to transform the lives of patients with severe heart failure.
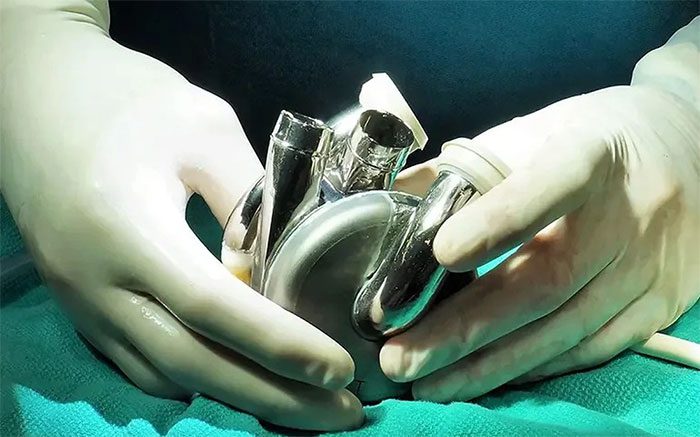
Fully Artificial Heart BiVACOR (TAH).
The Australian Minister for Health and Aged Care, Mark Butler, recently announced a funding grant of AUD 50 million (equivalent to over 800 billion VND) from the Medical Research Future Fund (MRFF) for the Artificial Heart Research Program (AHFP) led by Monash University.
Based at the Monash Alfred Baker Cardiovascular Research Centre at The Alfred, the AHFP is an initiative aimed at developing and commercializing three implantable devices, including a mini pump, a left ventricular assist device (LVAD), and a total artificial heart – which can replace a human heart entirely.
These devices target the most common forms of heart failure, promising to halve the number of heart failure-related deaths in Australia.

Monash University (Australia) has received AUD 50 million from the Medical Research Future Fund to develop new heart implant devices that will transform the lives of those suffering from severe heart failure.
The three groundbreaking heart implant devices that Monash University will develop include:
- Mini Pump: A miniaturized device implanted in patients with limited treatment options for heart failure symptoms, particularly those with preserved ejection fraction (HFpEF). By relieving left atrial pressure and reducing pulmonary pressure, this mini pump provides a unique solution for HFpEF, addressing a significant portion of heart failure cases with no other treatment options.
- New Left Ventricular Assist Device (LVAD): An improved device implanted alongside the natural heart to assist blood pumping function.
- Fully Artificial Heart BiVACOR (TAH): An advanced device designed to completely replace a failing natural heart.
This device employs cutting-edge technology, including an optimized hydraulic system that supports both sides of the heart, a strong magnetic levitation (MAGLEV) drive that enhances durability and biocompatibility, and an automatic flow adjustment that responds to patient needs without user intervention.
Additionally, it serves as foundational technology for advanced LVAD devices.
These three devices incorporate revolutionary technologies that mimic the functions of a natural heart, automatically responding to the physiological demands of the body. This advancement marks a significant improvement over current devices, which operate at a fixed blood flow rate, limiting patients’ physical activity.
Professor David Kaye – Director of Cardiology at The Alfred and head of the Monash Alfred Baker Cardiovascular Research Centre, shared: “The average survival rate for heart failure patients is comparable to that of certain cancers, with only about 5 years and even less for those with advanced heart failure. These patients will benefit the most from the devices we are developing.
This is the first time we are applying the method of providing physiological responses automatically to an implantable heart device, significantly improving patients’ quality of life, allowing them to perform daily activities without breathlessness.”

Professor David Kaye – Director of Cardiology at The Alfred, head of the Monash Alfred Baker Cardiovascular Research Centre (center).
The technological advances being developed by the AHFP are not limited to the three devices mentioned above but have also begun on a range of external devices such as: wearable controllers, infection-resistant power transmission, smart clinical advisor for healthcare professionals, mobile applications and websites for patients, online feedback portals for clinicians, customizable wearable devices for patients, advanced surgical tools, and clinical training platforms for surgeons.
Clinical trials for this trio of devices will soon be conducted at leading hospitals in Australia. Accordingly, The Fully Artificial Heart BiVACOR (TAH) is expected to be brought to market by 2025, followed by the New Left Ventricular Assist Device (LVAD) in 2029, and the mini pump by 2031.
By 2036, the project is expected to generate AUD 1.8 billion for Australia and the Australian society, including savings for the healthcare system, expanding the local industry in research and manufacturing, creating over 2,000 jobs, and helping Australians gain early access to clinical trials and new life-saving technologies.