Chinese scientists have developed a wireless energy receiving and storage device that is biodegradable and capable of powering biocompatible electronic implant devices, such as completely biodegradable drug delivery systems.
According to the South China Morning Post (Hong Kong, China), biocompatible electronic systems—such as monitoring sensors and implantable drug delivery systems—are minimally invasive and reliable methods for effectively monitoring and treating diseases. However, a study published in the journal Science Advances by researchers from Lanzhou University indicates that the development of energy modules to operate these devices is lagging behind the creation of biodegradable sensors and circuits.

The new wireless power supply system, which is biodegradable, may help operate future drug delivery implant devices. (Photo: Shutterstock).
Although scientists have developed various biodegradable power supply devices, they are typically single-use and do not provide sufficient power for biomedical applications.
Furthermore, power supply devices are often connected to skin chargers that can cause inflammation. Devices powered by non-rechargeable batteries require surgical replacement, which can lead to complications.
To address these shortcomings, researchers have developed a wireless implantable power system with high energy storage efficiency and biocompatibility characteristics. The device’s soft design and flexibility adapt to the shape of human tissues and organs.
This wireless power supply device includes a magnesium coil that charges the device when an external transmission coil is placed over the skin above the implant. The energy received by the magnesium coil flows through a circuit before entering an energy storage module formed by zinc-ion hybrid supercapacitors. These supercapacitors store energy in the form of electrical energy, while batteries store energy in the form of chemical energy.
According to the research, although supercapacitors store less energy, they have a high energy density and can consistently release a significant amount of energy.
The prototype power supply system—embedded in the implant like a flexible biodegradable chip—can integrate energy reception and storage for the device. Consequently, electricity can flow directly through the circuit to an accompanying biocompatible electronic device or into the supercapacitor to ensure stable and reliable power output after charging.
Both zinc and magnesium are essential for the human body. Researchers emphasize that the amounts of zinc and magnesium in this device are below daily consumption levels, making the biodegradable implants biocompatible.
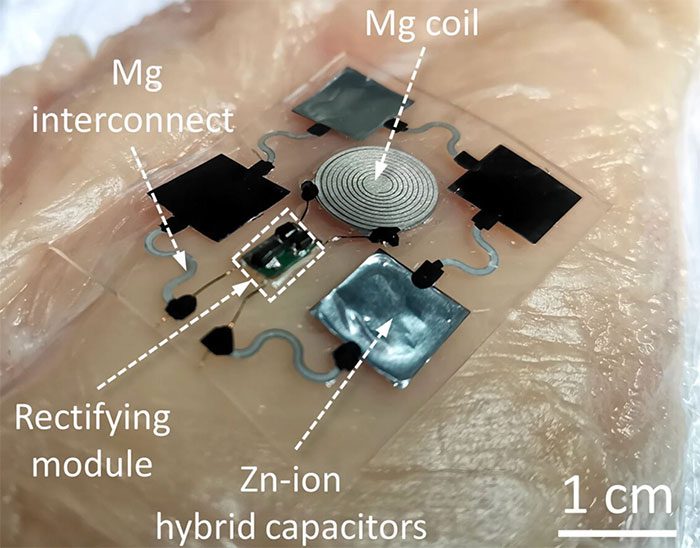
The wireless energy storage and supply device created by a team of Chinese scientists is biodegradable. (Photo: Lanzhou University).
The entire device is wrapped in polymer and wax, allowing it to bend and twist according to the structure of the tissue.
Tests on mice showed that this device could operate effectively for up to 10 days and completely degrade within two months.
The operational duration of this device can be adjusted by varying the thickness and chemical properties of the coating.
Scientists emphasize that the drug delivery system can be integrated into various tissues and organs in the body, playing an important role in localized drug delivery and treatment.
To demonstrate the functionality of the power source, researchers connected stacked supercapacitors to a receiving coil and a biodegradable drug delivery device, then implanted it into mice. The prototype consisted of separate pieces joined together.
The drug delivery device, containing anti-inflammatory medication, was implanted into mice suffering from fever caused by yeast.
As a result, during a 12-hour observation period, the temperature of the non-implanted mice was significantly higher than that of the implanted group.
However, researchers noted that the on-off functionality of the device still has limitations, as the device only stops when the power runs out. They suggest that regulated charging activation could help control the timing of the device’s operation.
In mice implanted with the device that was not charged, researchers also observed some passive drug release, as the recorded temperature in this group also decreased compared to the control group.
Scientists stress that this prototype “represents a significant advancement in promoting a range of temporary implantable bioelectronic devices with the potential to provide effective and reliable energy solutions.”




















































