Breakthrough Potential in Energy Storage Technology Discovered
Rechargeable lithium-ion batteries power countless electronic devices in modern times. They are descendants of lithium metal batteries, which have not been widely developed due to significant concerns. Manufacturers have been hesitant for a good reason: while lithium metal batteries have the potential to store energy twice as much as lithium-ion batteries, they also carry a much higher risk of fire and explosion.
A new study conducted by the California NanoSystems Institute at the University of California, Los Angeles (UCLA) has unveiled significant findings that could allow lithium metal batteries to surpass their lithium-ion counterparts. The research was published in the prestigious scientific journal Nature.

The UCLA research team has managed to prevent lithium from corroding, forming neat polyhedral structures – (Photo: UCLA).
Lithium metal is highly reactive with chemicals, to the extent that when placed under normal conditions on a flat surface, corrosion occurs immediately. However, the UCLA research team successfully developed a technique to prevent corrosion, and surprisingly, they discovered that lithium atoms formed a unique shape – a twelve-faced polyhedron known as rhombic dodecahedron.
“Currently, there are thousands of studies surrounding lithium metal, most of which describe different structures, whether they are ‘clunky’ or ‘cylindrical,” said study author Li Yuzhang. “It was surprising for us to find a way to prevent surface corrosion, and instead of the shapes [previously recorded], we observed a polyhedron that had long been predicted to exist. Ultimately, this research allows us to reevaluate our understanding of lithium metal batteries.”
In other words, Yuzhang’s team has found a way to prevent lithium from corroding while simultaneously discovering a lithium structure that makes future batteries less likely to short circuit.
At the microscopic level, lithium-ion batteries can store positively charged lithium atoms within a carbon structure. In contrast, lithium metal batteries coat the electrodes with lithium, which requires ten times more lithium material than lithium-ion batteries. The increased lithium content accompanies both high performance and a higher risk of fire and explosion.

Lithium-ion batteries come with the risk of explosion.
The process of integrating lithium into electrodes involves a 200-year-old technique that uses electricity and a salt-containing solution known as an electrolyte. Typically, lithium forms at the microscopic level, creating root-like structures that spread in multiple directions. In a battery, if two lithium strands touch, a short circuit occurs, potentially causing the battery to explode.
In the new experiment, lithium appears in its pure form when corrosion layers are absent. UCLA scientists note that this indicates that lithium metal batteries are now “more docile,” as the lithium atoms are neatly arranged rather than extending in multiple directions. This new finding could lead to a breakthrough in technology, particularly in the energy storage sector.
“Scientists and engineers have spent two decades compiling data on how to shape metals like gold, platinum, and silver into cubes, spheres, and cylinders. Now we have learned more about the shape of lithium,” researcher Yuzhang commented. “We must now ask whether we can arrange them into blocks, enhancing both performance and safety of batteries.”
Until now, the prevailing belief has been that the electrolyte determines the shape of lithium structures. However, UCLA researchers have demonstrated that this is not the case.
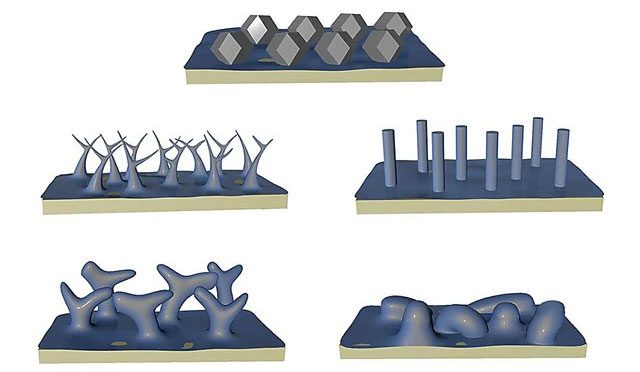
The image above shows how lithium forms when there is no corrosion layer, while the four images below depict lithium formation under normal conditions – (Photo: UCLA).
“We wanted to see if we could introduce lithium into the battery system faster than the corrosion rate,” said lead author Yuan Xintong in an interview. “In this way, we could observe how lithium disperses without being affected by the corrosion layer.”
The team successfully developed a lithium pumping technique that exceeds the corrosion rate, while witnessing lithium crystallizing into tiny, neat polyhedral shapes, with structures measuring just two millionths of a meter long, or the average length of a bacterium. The research team was able to observe the lithium structure using a cold electron microscope, which is used to determine the shapes of proteins and viruses.
According to Yuzhang, the lithium pumping technique is not yet optimized. However, the research team has accomplished the most challenging task: eliminating the corrosion factor of lithium and witnessing a previously unseen, neat lithium structure. This discovery could lead to safer lithium-based energy storage technology in the future, and a new, safer battery system could elevate the industry to new heights.