While other carbon capture systems operate in laboratories using pure CO2 from pressure vessels, the artificial leaf developed by engineers at the University of Illinois Chicago functions in the real world. It captures CO2 from more diluted sources such as the atmosphere, emissions produced by coal-fired power plants, and releases it for use as fuel and other materials.
This leaf captures CO2 from more diluted sources such as the atmosphere and emissions… (Photo: Meenesh Singh)
“Our artificial leaf system can be deployed outside the laboratory, where it has the potential to play a significant role in reducing greenhouse gases in the atmosphere, thanks to its high carbon capture rate, relatively low cost, and moderate energy requirements compared to even the best laboratory systems,” said Meenesh Singh, assistant professor of chemical engineering at the University of Illinois Chicago (UIC) and the second author of the study.
Using a previously researched theoretical concept, the scientists modified a standard artificial leaf system with inexpensive materials, including a water gradient with one dry side and one wet side across a charged membrane.
On the dry side, an organic solvent binds to available CO2 to produce sodium bicarbonate (NaHCO3, also known as baking soda) on the membrane. As bicarbonate forms, negatively charged ions are pulled through the membrane toward the positively charged electrode in the aqueous solution on the wet side of the membrane. The liquid solution dissolves bicarbonate back into CO2, allowing it to be released and harnessed for fuel or other uses. Electricity is used to accelerate the transport of bicarbonate across the membrane.
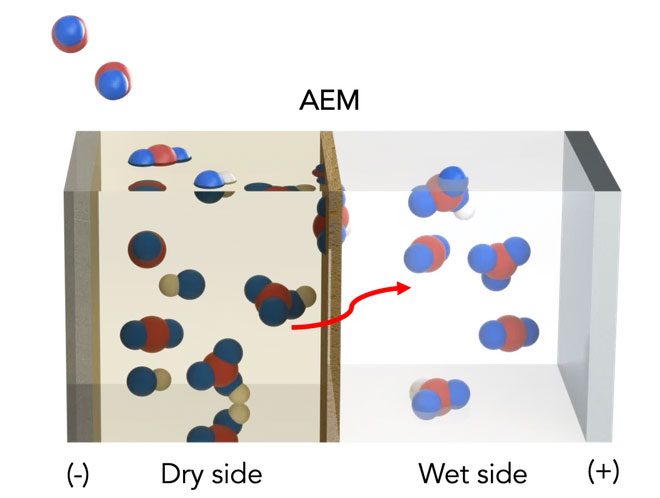
The artificial leaf system for capturing CO2 consists of both wet and dry sides, as designed by UIC scientists. Red represents carbon atoms, blue represents oxygen, and white represents hydrogen (Photo: UIC)
The artificial leaf system is designed to be small enough to fit into a backpack. When testing the system, UIC scientists found that it has a very high throughput. The carbon capture rate per unit surface area required for the reactions is 3.3 millimoles/4cm2 per hour. This metric is over 100 times higher than other systems, while requiring only a modest amount of electricity (0.4 KJ/hour) to power the reaction, less than the energy needed to run a 1-watt LED bulb. The estimated cost per ton of CO2 is $145, complying with the U.S. Department of Energy’s recommendation that costs should not exceed $200 per ton.
“What is particularly exciting is that the real-world application of the electrically driven artificial leaf with high throughput and small modular surface area means we can stack the leaves, adding or removing modules to better meet demand and be used efficiently in homes and classrooms, not just in profit-driven industrial organizations. A small artificial leaf module the size of a household humidifier can remove over 1kg of CO2 per day, and four stacks of industrial electrodialysis can capture over 300kg of CO2 per hour from emissions,” Singh shared.
Electrodialysis (ED) is the process of passing an electric current through a semipermeable membrane to separate molecules in a solution. Electrodialysis is commonly used to desalinate brackish or saline water.
The design of the artificial leaf and the experimental results from UIC scientists were recently published in the international scientific journal Energy & Environmental Science in its January issue this year (2022).