The transplantation of pig hearts into humans promises to address the organ shortage crisis, but it also raises numerous ethical questions.
For cardiac surgeon Brandon Guenhart from Stanford University School of Medicine, the most critical factor in organ transplantation is time.
In the case of Mr. P., the anesthesiologists placed him in a deep coma after the resuscitation team reported that the donated heart had arrived. Two surgeons began the operation an hour before the heart was transported from the airport to the hospital. This is a typical heart transplant procedure.
On January 7, at the University of Maryland Medical Center, 57-year-old patient David Bennett received a heart transplant from a special donor: a genetically modified pig.
In 2021, 41,354 organ transplants from human to human were performed, yet over 100,000 Americans are still waiting for transplants. Every day, 17 people die because they cannot wait any longer.
The Line Between Humans and Animals
Xenotransplantation (the transplantation of cells, tissues, and organs from one species to another) promises to alleviate this severe shortage and reshape the concept of human longevity.
However, the barrier between humans and animals remains significant. The SARS-CoV-2 virus, which originated from animals, has claimed the lives of over 6 million people, so crossing this boundary could lead to many disasters.
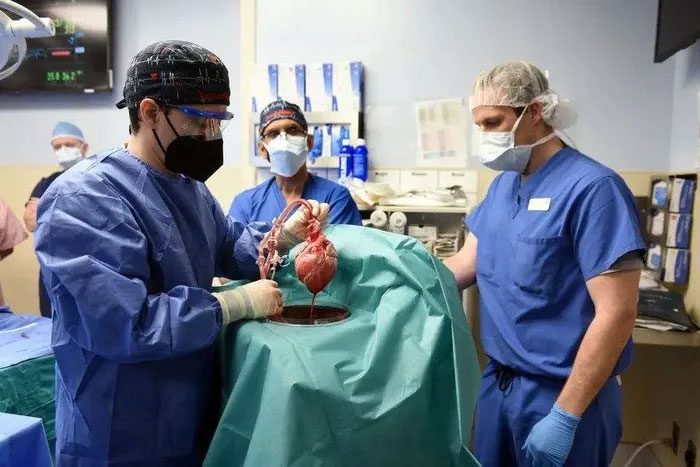
Dr. Muhammad M Mohiuddin places a genetically modified pig heart into a storage device before transplanting it for Mr. Bennett. (Photo: Reuters).
Humans are a species of animal. But animals are not humans. Nevertheless, humans have created hybrid cultural innovations. Egyptian mythology features the god Horus depicted with a hawk’s head, and the goddess of war Sekhmet has a lion’s head.
Similarly, the Hindu god Ganesha was beheaded and revived with an elephant’s head. In ancient Greece, mythical creatures appeared in stories, from the Minotaur with a bull’s head to the snake-haired Medusa.
The International Xenotransplantation Association has chosen Lamassu as its mascot. This is an Assyrian deity with the body of a bull, wings of a bird, and the head of a man.
Xenotransplantation initially involved only the transplantation of cells and tissues. In France and England during the 17th century, blood was transfused from animals to humans to treat diseases.
This transplantation method was improved to include bone, cornea, and skin grafts. The most famous case was French surgeon Serge Voronoff, who transplanted slices of testicles from chimpanzees and macaques into men and ovaries from macaques into women.
While cell and tissue transplants have been around for a long time, organ transplants are more challenging. It is very difficult to stitch all the blood vessels together. In 1912, French surgeon Alexis Carrel won the Nobel Prize for his research on suturing vascular systems, addressing this issue.
Half a century later, in 1964, surgeon James Hardy from the University of Mississippi performed the world’s first heart transplant, placing the heart of a chimpanzee named Bino into the chest of 68-year-old patient Boyd Rush. Rush survived only 90 minutes, as the chimpanzee’s heart could not sustain his life.
Then there was Baby Fae, an astounding case of xenotransplantation. She was a 12-day-old infant with hypoplastic left heart syndrome. In 1984, surgeons at Loma Linda University in California transplanted a walnut-sized heart from a macaque into Baby Fae.
After the surgery, her condition initially improved. However, she died 21 days later due to her immune system reacting against the grafted heart tissue. This sparked outrage from animal rights activists and bioethicists.
The Controversy Over Pigs
Pigs do not have a good reputation. Judaism and Islam prohibit the consumption of pork. In the epic Odyssey, the sorceress Circe transforms Odysseus’s gluttonous crew into pigs.
However, pigs are actually very intelligent animals capable of expressing emotions. They are considered smarter than dogs and chimpanzees based on various IQ tests.

Pigs at the Badersfeld experimental farm in Oberschleissheim, Germany. (Photo: Reuters).
After the failure of Baby Fae, primates fell out of favor, and pigs became the new focus for researchers. They argued that pigs are easy to transport, can be raised in sterile environments to reduce infection, and can provide the necessary organ sizes.
This is a valid point, but Dr. Brad Bolman, a historian at the University of Chicago, argues that sheep, goats, or other animals could also be suitable. However, scientific arguments have been constructed to convince that pigs are the right choice.
According to Bolman, pigs were chosen for economic and social convenience. They reproduce quickly and mature in just six months. Pigs fall outside the scope of the Animal Welfare Act, being common agricultural animals worldwide.
The PETA organization has deemed the transplantation from pigs to humans as “unethical, dangerous, and a colossal waste of resources,” asserting that “animals are not tools for transformation but intelligent beings.”
Xenotransplantation experts often dismiss ethical concerns by referencing the pork industry. After all, if pigs are raised for meat, raising pigs for science has a nobler purpose.
Dr. Bolman states: “Science consumes animals, whether they are edible or not. In short, science is a carnivore.”
Gene Editing in Pigs
Conducting xenotransplantation requires selective humanization of a pig. Therefore, we cannot simply transplant a pig’s heart into a human body, according to Dr. Richard Pierson, Director of the Transplantation Science Center at Massachusetts General Hospital.
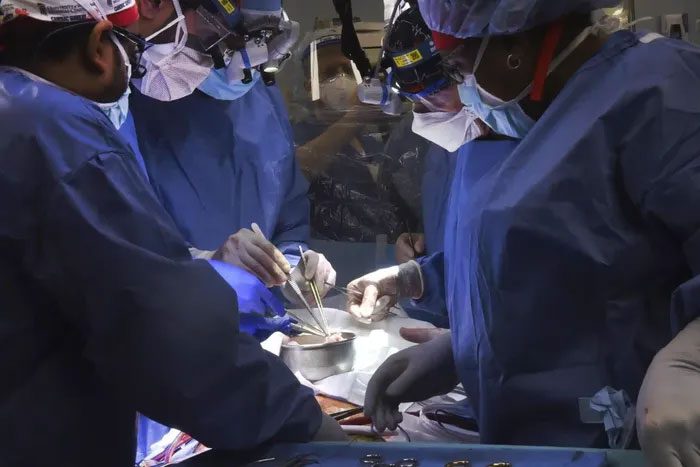
Doctors performing a pig heart transplant for Mr. David Bennett in Baltimore. (Photo: AP).
The biotechnology company Revivicor in Virginia has used CRISPR gene editing technology to create a special line of pigs with 10 modifications. Four genes were removed, and six genes were added. The “recipe” for “preparing a pig heart” for humans includes:
Removing three genes specific to pigs.
Removing one growth hormone gene to prevent the pig heart from overgrowing in the new body.
Adding two complement-inhibiting genes to prevent antibodies from destroying the pig heart and two anticoagulant genes to prevent the patient’s blood from clotting within the foreign organ.
Adding two anti-inflammatory genes to prevent swelling of the heart. One of these genes signals to the immune system that the pig heart is a friend (belonging to it), not food (not belonging to it).
After this series of “cut and paste,” the next challenge is to keep the pig “clean.” No one wants to transplant a pig heart filled with viruses, bacteria, and parasites that could infect humans. Therefore, these pigs will be raised in a sterile environment, without going outside.
With this “bubble pig” farming method, any foreign or external viruses have been eliminated. Thus, these pig hearts are safe to transplant into humans.
However, this is not entirely the case. Mr. Bennett’s heart still tested positive for porcine endogenous retrovirus (PERV).
Regardless of whether there are infectious viruses, Dr. Pierson does not see this as a significant barrier to xenotransplantation. HIV treatment drugs seem quite effective against viruses, and the Boston-based biotechnology company eGenesis has even created a pig with 60 genes devoid of PERV.
Mr. Bennett lived only 60 days after the surgery. He may have contracted a pig virus. Thoracic surgeon Bartley Griffith believes the pig virus Mr. Bennett contracted was not PERV, but an external virus called porcine cytomegalovirus (pCMV). It is possible that pCMV went undetected during the assessment process of that pig.
Ultimately, there are no boundaries between humans and animals. Pigs share 98% of their genetic makeup with humans. Besides the ethical issues, humans cannot ignore the potential of xenotransplantation. If this is feasible, we must create a new livestock industry where pigs are bred and slaughtered to sustain human life.
Dr. Chris Walzer, Executive Director of the Wildlife Conservation Society, believes that pig organ transplantation could benefit from One Health—a collaborative approach across health, veterinary, and environmental sectors to achieve optimal health for all species.
All of this is part of a shared ecosystem. And it would be very dangerous if this connection were to break.



















































