Cyanide has a long-standing history as a leading poison in cases of poisoning and murder. Over the centuries, its danger has not diminished.
What is Cyanide?

The use of poison as a weapon is a skill that humans have mastered since prehistoric times.
Millions of years ago, when prehistoric hunters first dipped their sharpened arrows into snake venom, they discovered a weapon that could change the course of history with just a minuscule amount, like the tip of a needle: poison.
Throughout many centuries, dozens of different deadly poisons have become prevalent. We can mention those derived from plants such as poison hemlock, belladonna, aconite, foxglove, and opium…
However, in modern warfare, particularly since the 20th century, no poison has gained as much notoriety as cyanide, frequently referenced in cases of poisoning and murder.
The term “cyanide” originally referred to any chemical containing a carbon-nitrogen (CN) bond. However, it has gradually become synonymous with the poison cyanide.
According to the U.S. Centers for Disease Control and Prevention (CDC), cyanide can exist as a colorless gas, such as hydrogen cyanide (HCN) or cyanogen chloride (CNCl). Additionally, it can also be found in crystalline forms like sodium cyanide (NaCN) or potassium cyanide (KCN).
A certain amount of cyanide can be found in foods such as cassava, lima beans, almonds… or the seeds of common fruits like apricots, apples, and peaches…
In other words, within the “gifts” that nature bestows upon humanity lies a weapon that can take lives in an instant.
In fact, many people have died from consuming cassava due to the cyanide it contains. Moreover, cyanide is not too difficult to produce and can be sourced from readily available raw materials.
In manufacturing, cyanide is quite commonly used in the production of paper, textiles, plastics, and metallurgy. Additionally, gaseous cyanide is used to eliminate pests and harmful insects.
“The King of Poisons”

French soldiers wearing gas masks, using poisoned bayonets during the Ypres battle in April 1915 (Photo: Hulton).
Cyanide is an extremely toxic chemical, even listed among the deadliest poisons.
The use of cyanide as a poison traces back to the Roman Emperor Nero (37-68 AD) when he used cherry laurel water containing cyanide as a poison.
Cyanide was also used during the Franco-Prussian War (1870-1871). There, Emperor Napoleon III urged his troops to dip their bayonets into cyanide poison to easily take down the enemy.
Cyanide was used as a poison for suicide during World War II and the Cold War. The most common forms were solid potassium cyanide, which dissolves in water, and liquid distilled hydrogen cyanide.
The suicide tactic with poison was employed by many Nazi leaders towards the end of World War II. Notably, Adolf Hitler and his wife Eva Braun committed suicide with cyanide in a bunker in Berlin.
Subsequently, Hermann Göring, a senior leader of the Nazi regime, also chose to take his own life to avoid execution by biting into a cyanide capsule hidden in his mouth.

Adolf Hitler (right) committing suicide with cyanide (Photo: Telegraph).
Since then, cyanide has become almost synonymous with suicide poison in films featuring detectives and spies.
Many literary works recount that government intelligence agencies also provided their agents with “L” poison (with “L” standing for “lethal”). This was done to prevent agents from being tortured and revealing state secrets if captured by the enemy.
This poison is primarily composed of cyanide, hidden within secret compartments of glasses or pens. When agents chew and swallow these items, the poison is released, ending their lives in an instant.
Why is Cyanide Dangerous?
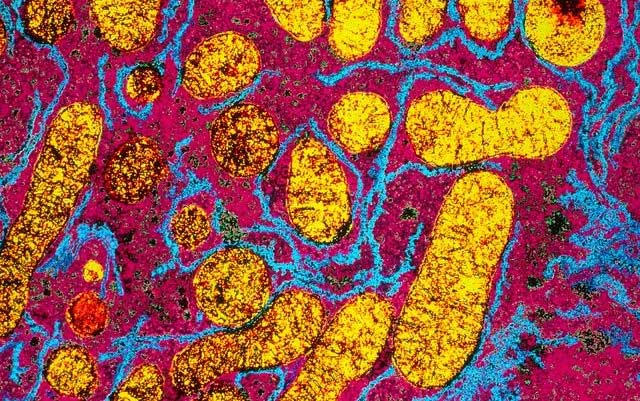
Cyanide attacks mitochondria and the electron transport chain in cells (Photo: Getty).
According to the CDC, the reason cyanide is highly lethal is due to its ability to inhibit the body’s utilization of oxygen. Specifically, cyanide attacks the very cells that sustain life, namely mitochondria and the electron transport chain – known as the “power plants” of the cell.
Mitochondria are responsible for cellular respiration and produce energy – called adenosine triphosphate (ATP) – from oxygen. To do this, mitochondria require a specific enzyme called cytochrome oxidase.
However, when the body is exposed to cyanide, cyanide ions bind to cytochrome oxidase, preventing it from performing its life-sustaining functions.
When cells can no longer utilize oxygen, the situation begins to deteriorate. The body gradually progresses towards respiratory failure or heart failure within just a few minutes.
Yet, the most dangerous aspect of cyanide is that it has many pathways to reach its victims. Cyanide exposure can occur through skin contact, eyes, or inhalation through the respiratory tract. Additionally, ingestion of cyanide in solid or liquid form can also be toxic.

Cyanide’s high lethality is due to its ability to inhibit the body’s utilization of oxygen.
Typically, after cyanide poisoning, victims will go through 3 stages.
- The first is the agitation stage, where the victim shows signs of anxiety, agitation, rapid breathing, and confusion.
- Then, the victim begins to experience seizures, difficulty breathing, low blood pressure, and reduced ventilation.
- Finally, the victim gradually enters a state of muscle flaccidity and loss of reflexes, suffers cardiovascular collapse, decreased blood oxygen levels, leading to death.
Some signs to identify cyanide poisoning include headaches, dizziness, nausea, rapid breathing, increased heart rate, restlessness, and exhaustion.
Upon noticing these signs, the most feasible action is to quickly take the cyanide-poisoned victim to the nearest medical facility for timely emergency treatment. At this point, time is a “lifesaving” factor, as the victim needs medical treatment as soon as possible.
According to health organizations, if a person poisoned by cyanide is not treated within 2 hours, the risk of death is very high. Even those who survive significant exposure to cyanide face the risk of severe heart, brain, and nerve damage.
Vietnamese law does not classify cyanide as a prohibited chemical. However, the trade of cyanide must comply with the Chemical Law of 2007 and Decree 113, meaning that to trade cyanide, one must have a special license, and to purchase it, one must have a referral letter and documentation specifying the quantity and purpose of the transaction.


















































