One of the most peculiar aspects of Uranus and Neptune is their magnetic fields. Each of these planets possesses a strong, off-center magnetic field that is tilted away from their rotation axes in a way that has never been observed in any other planet.
Until now, we did not fully understand why this is the case. Now, a group of researchers from China and Russia has provided us with a new piece of the puzzle: an ionized form of water, a truly strange substance called aquodiium that may exist deep within the extremely high-pressure environments of the ice giants.
Aquodiium consists of a regular water molecule with two additional protons, giving it a positive charge. When present in sufficient quantities, they can generate a planetary magnetic field similar to that of Uranus and Neptune.
The planetary magnetic field extends into the surrounding space of the planets that generate it. However, they are produced deep within the planet by moving charges, although the exact mechanisms may vary.
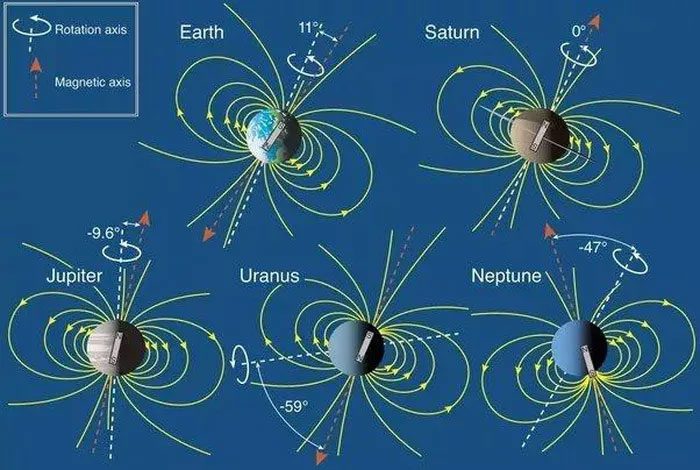
Magnetic fields of Earth, Saturn, Jupiter, Uranus, Neptune.
On Earth, molten iron-nickel flows around the core, spins, convects, and conducts electricity, converting all that kinetic energy into an electron flow similar to that in a gigantic generator beneath the surface. For Jupiter and Saturn, scientists believe that it is the hydrogen under immense gravitational pressure that transforms into a metallic state to create the magnetic field.
Earth, Jupiter, and Saturn have relatively neat magnetic fields resembling that of a giant bar magnet aligned along the planet’s axis, with its magnetic field lines forming a shape akin to a cage connecting the north and south poles.
In contrast, the magnetic poles of Uranus and Neptune are tilted 59 and 47 degrees, respectively, away from their rotation axes, and their magnetic field lines continuously change and shift. And they do not actually concentrate around the planet’s core like Earth and the two gas giants.
A possible explanation is that the magnetic field could be generated by an ionic conducting fluid, where ions act as charge carriers rather than the fluid serving as a conduit for electrons.
Physicist and chemist Artem Oganov from the Skolkovo Institute of Science and Technology in Russia explains: “Hydrogen surrounding the rocky core of Jupiter under high-pressure conditions is a form of liquid metal: it can flow, much like molten iron flows inside Earth, and its conductivity is due to the free electrons shared by all the hydrogen atoms squeezed together. In Uranus, we think that the ions of hydrogen or protons themselves are the charge carriers.”
The question then arises: which ions? Some, like ammonium, are obvious possibilities. But could the water molecules of the planets play a more significant role in this process?
A team of researchers led by physicist Jingyu Hou from Nankai University (China) revisited the fundamental principles combined with models of how molecules could evolve, delving into a concept known as chemical hybridization.
This is when the orbital elements of an atom mix or combine to create an atom that can bond in new ways. There are various types of hybridization, but the relevant type here is sp3 hybridization, where four orbitals form a tetrahedral arrangement around a central nucleus.
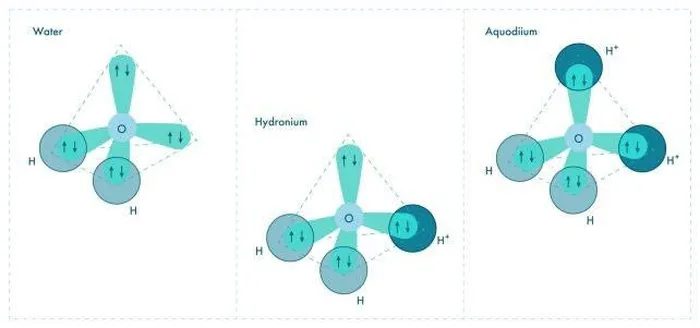
Structural representation of water molecule, hydronium, and aquodiium.
Each point in the tetrahedron has a single electron capable of bonding with another atom or a pair of electrons that cannot bond with other atoms.
Oxygen has two unpaired electrons and two pairs of electrons in its outer shell. If you attach a hydrogen atom to each available valence electron, you will obtain H2O – water.
Sometimes hydrogen, which lacks an electron – having only a nucleus with 1 proton – will attach to one of the electron pairs to form a molecule known as hydronium.
Physicist Xiao Dong from Nankai University analyzes: “The question is: Can you add another proton to the hydronium ion to fill the missing part? Such a configuration under normal conditions is very unfavorable in terms of energy, but our calculations show that there are two conditions that could make this happen.”
“First, high pressure forces matter to reduce its volume, and sharing the previously unused oxygen electron pair with the hydrogen ion (proton) is a neat way to do it: similar to a covalent bond with hydrogen, except both electrons in the pair come from the oxygen atom. Second, you need a lot of available protons to create an acidic environment, as the effect of an acidic environment is to provide protons.”
The researchers conducted computational modeling, and under conditions similar to those believed to exist within Uranus and Neptune, this is what occurred. At temperatures around 3,000 degrees Celsius and pressures of 1.5 million atmospheres, protons bonded with hydronium to form H4O – aquodiium.
Of course, this hypothesis remains theoretical. More detailed observations of the two outermost planets in the Solar System will be needed to further investigate this possibility; however, these findings provide us with a new way to understand the peculiarities of the two blue planets, Uranus and Neptune.





















































