A small experiment has shown that after being nourished via internal jugular vein, a tiny device “controlled by thought” can record brain activity from a nearby blood vessel, thus allowing doctors to avoid craniotomy.
The device, named Stentrode, is designed to enable paralyzed individuals to operate assistive technologies solely through their thoughts. For instance, participants in the trial used this device to create text messages and emails, as well as conduct banking transactions and shop online, according to a report published on January 9 in JAMA Neurology.
Initial data from the trial was also presented in March 2022 at the 74th Annual Meeting of the American Academy of Neurology in Seattle.
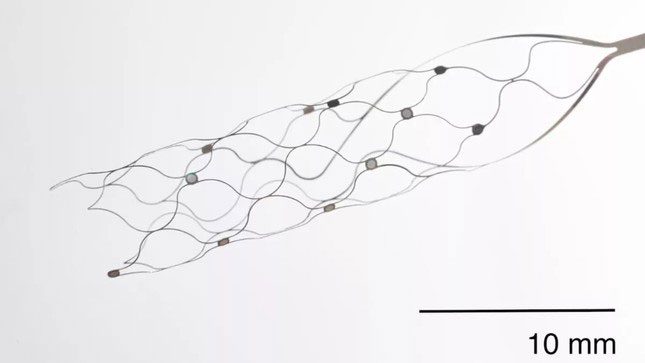
A new brain-computer interface device that does not require open-brain surgery for implantation.
While other thought-reading devices designed for similar purposes are typically placed on or inside the brain during open surgery, doctors can implant the Stentrode without needing to open the patient’s skull, the trial organizers wrote in their report.
They stated: “The blood vessels of the brain provide a less invasive pathway to reach the motor cortex,” an area on the wrinkled surface of the brain related to motor control.
Previously, the research team demonstrated that the Stentrode could be used in animals to record signals from the brain and transmit electrical stimulation to organs, according to the Royal Melbourne Hospital in Australia, an organization involved in the trial. The recent human clinical trial – known as the Stentrode With Thought-Controlled Digital Switch (SWITCH) study – is the first study to test this device on humans.
The trial involved four Caucasian men diagnosed with amyotrophic lateral sclerosis (ALS), a progressive disease that causes the voluntary control neurons to degenerate. At the time of the trial, all participants were severely paralyzed in their upper limbs, had varying degrees of lung function, and experienced impaired speech.
Each participant had the Stentrode placed in their superior sagittal sinus, a large vein that drains fluid from the brain into the jugular vein and is adjacent to the motor cortex. The device itself is made of a mesh material containing 16 electrodes.
According to a statement in March 2022, doctors insert the device into the body using a catheter, and once it’s in the correct position, they expand the mesh to lay it against the wall of the sinus. Synchron, the brain-computer interface (BCI) company behind Stentrode, has a wire running from the electrodes to a small electronic device in the chest, which wirelessly transmits the recorded brain signals to a computer.
“All patients tolerated the procedure well and were usually discharged home within 48 hours,” said Dr. Peter Mitchell, principal co-investigator and director of interventional neurology at the Royal Melbourne Hospital, in a separate statement. Only one of the four patients stayed in the hospital an extra day before being discharged.
The most common side effects were headaches and bruising at the incision sites, and no one experienced serious side effects during or after the procedure. Over a one-year follow-up period, none of the participants suffered from blood clots (thrombosis); vascular obstruction; device “movement,” meaning shifting of the device within the body; or any other serious device-related adverse effects that could lead to death or permanent disability.
Furthermore, “The BCI maintained a stable signal throughout the study and all participants successfully controlled the computer using the BCI,” reported the authors.
The research team concluded: “Data on safety and feasibility from the first human study indicate that it is possible to record neural signals from blood vessels and favorable safety profiles may promote broader and faster BCI translation for those who are paralyzed.”


















































