The human body is a fertile ground for various horrifying parasitic “monsters”. They feed, reside, reproduce, and then return to harm us.
The Horrors of Parasitic Species in the Human Body
1. Botfly Larvae – Burrowing Under the Skin
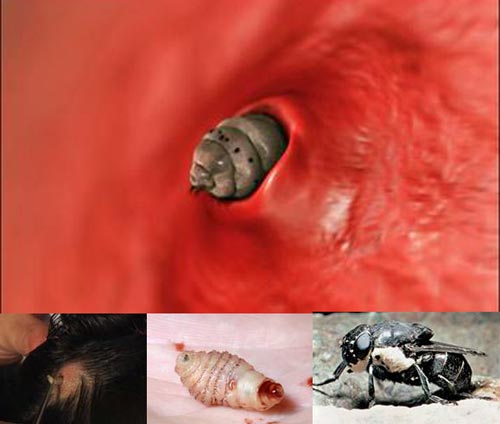
Botflies belong to the family Oestroidea, primarily found in Mexico, Central, and South America. Their life cycle begins when a female botfly abducts and lays eggs on the body of a blood-sucking arthropod, such as a mosquito or flea. This intermediate host is then released to continue feeding on other animals, often livestock and humans.
As a result, the botfly eggs transfer to the host. They react to temperature changes when moving from the intermediate host to the primary host, hatching into larvae. These larvae burrow in and feed on the host’s blood beneath the skin through the bite of the intermediate host or through hair follicles. The area where they reside becomes swollen, secreting pus and fluids that cause pain.
The larvae remain in the host for about 12 weeks until they grow sufficiently. Afterward, they crawl out, fall to the ground, and develop into pupae in the soil, where they cocoon until becoming mature botflies, starting a new life cycle.
Treatment: The usual method is to remove the parasitic larvae from the host through surgical means or simply by squeezing the wound to push the larvae out.
Prevention: Apply mosquito repellent, wear protective clothing, and sleep under a mosquito net to avoid bites from mosquitoes or fleas.
2. Schistosoma mansoni – Residing in the Brain
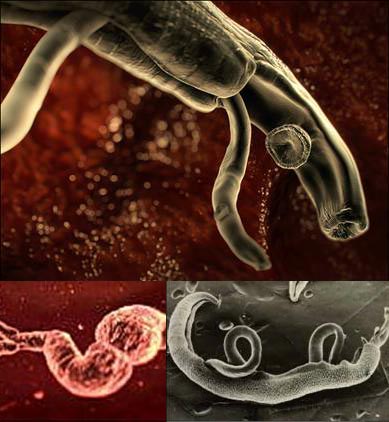
Schistosoma mansoni is a species of blood-fluke found in freshwater lakes in Africa, Asia, and South America. Their life cycle begins when an adult worm lays eggs in the blood vessels surrounding the intestines of the host. The eggs exit the host through feces. Upon contact with water, they hatch into tiny larvae. These larvae attach themselves to snails and burrow into their soft bodies. There, they develop into worm larvae, growing and eventually emerging from the snail to find another host with a blood supply.
When livestock or humans come into contact with this water, fatty acids on their skin attract the larvae, causing them to swim and attach to the host. They seek out open wounds or hair follicles, releasing a chemical that breaks down skin to create a tiny hole large enough for them to swim into the host’s body. Once inside, they suck blood and mature into adult worms, usually residing in blood vessels near the liver.
They mate in the blood vessels and lay eggs near the intestinal walls. The eggs attach to the host’s feces, starting a new life cycle.
Schistosomiasis is a prevalent disease in tropical regions, especially Africa. It is estimated that around 200 million individuals worldwide are infected with this type of fluke annually. It can exist in the host’s body for decades without detection, silently destroying the host’s organs such as the liver, intestines, lungs, and spleen. It can be deadly if the worms migrate to the brain.
In children, the disease can cause anemia, malnutrition, and affect learning ability. In severe cases, the worms can move to the brain, lay eggs, and reside in the brain or spinal cord, leading to complications, paralysis, or even death.
Treatment: The most common treatment is administering deworming medication containing Praziquantel, which paralyzes the worms.
Prevention: Avoid contact with contaminated water sources, especially sewage.
3. Bed Bugs – Blood Feeders

The life cycle of bed bugs begins with the violent mating act of the male, which pierces the female’s back with its reproductive organ to fertilize the eggs. The wound on the female heals within 24 hours, after which it begins to lay eggs; on average, it lays about 12 eggs per day, totaling around 500 eggs in a lifetime. Within 10 days, the eggs hatch into nymphs, which are immediately attracted to the warm blood of animals such as livestock or humans.
CO2 in human breath and body temperature are attractive factors for bed bugs. They use their proboscis to suck blood from unsuspecting hosts (typically while they are sleeping) (bed bugs are primarily nocturnal). After feeding, the bugs hide to digest for about 7 days, then re-emerge to feed again. They molt around 4 times before reaching maturity, mating, and starting a new life cycle.
Bites often result in itching and red, swollen welts. They can also cause restlessness and discomfort at night.
Treatment: Antihistamine or corticosteroid medications are often prescribed to reduce symptoms caused by bed bug bites. Antiseptic and antibiotic ointments are also used to prevent infection. After discovering bed bug bites, all clothing, bedding, and furniture should be washed thoroughly to eliminate them.
Prevention: Regularly clean clothing, bedding, furniture, and carpets.
4. Amoeba Parasites – Eating the Brain
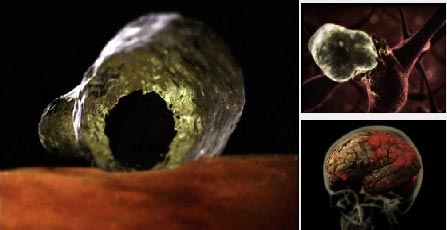
Naegleria fowleri is a type of parasite primarily found in freshwater and soil. Their life cycle begins in a cyst form attached to the bottoms of freshwater bodies. They enter the human body through the nose when exposed to water contaminated with amoebic larvae, especially when the host swims in unchlorinated freshwater like rivers, lakes, or pools. From the nose, they swim up to the brain and spinal cord, where they start to destroy brain tissue. When attacked by the immune system or when the environment becomes unfavorable, they can create a protective armor for defense. Once the danger has passed, they molt, begin to divide, and a new life cycle starts. They are heat-loving parasites, making infections more prevalent in the summer.
Amoebic parasites (Naegleria fowleri) often cause severe brain inflammation leading to death due to brain tissue destruction. Symptoms usually appear 2 weeks after infection, manifesting as headaches, fever, nausea, and stiff neck. In more severe cases, patients may experience dizziness, loss of balance, or even seizures and hallucinations. The disease progresses rapidly and can be fatal within 3-7 days.
Treatment: There is currently no specific medication available.
Prevention: Avoid contact with contaminated water. Use nose clips when swimming to limit exposure.
5. Wuchereria – Causing Elephantiasis

Wuchereria bancrofti is a common parasitic worm found in Southeast Asia. Its life cycle begins when an infected mosquito bites a host, releasing hundreds of larvae that travel into the bloodstream. They move to the lymphatic system, where they develop into adult worms measuring 7-10 cm in length. The adult worms produce countless larvae, which exit the lymphatic system and move to other organs in the body. During the day, the larvae reside in the lungs, and at night, when the host’s body temperature drops, they crawl out through the skin to be carried away by mosquitoes, beginning a new life cycle.
In severe cases, filarial worms cause elephantiasis – a condition characterized by massive swelling in the legs. Filarial worms can reside in the body for decades before being detected through respiratory symptoms (due to larvae accumulating in the lungs), often misdiagnosed as asthma. Over the years, the dead worms accumulate in the lymphatic vessels, causing swelling in the legs, pus formation, and fluid discharge.
Treatment: Anti-parasitic medications combined with thorough bathing and skin care. Exercise and massage can help alleviate pain.
Prevention: Apply insect repellent and use mosquito nets while sleeping.
6. Strongyloides stercoralis
Strongyloides is classified as one of the most dangerous digestive parasites yet is still categorized by the WHO as a neglected disease. Dr. Bui Tien Sy from the Microbiology Department at the 108 Central Military Hospital states that Strongyloides is considered the most dangerous among gastrointestinal parasites.
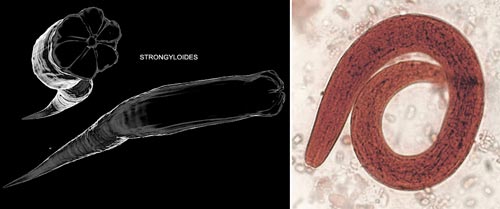
Strongyloides is a parasitic worm in humans that causes diseases related to the digestive system. In its larval stage, it appears thread-like and can exist independently in the soil without a host. Its life cycle begins when the larvae come into contact with a host through contaminated soil. The larvae then penetrate the skin, move to the pulmonary arteries, and subsequently migrate to the host’s respiratory organs, waiting to be swallowed into the intestines.
In the digestive system, they nestle and feed comfortably in the small intestine until they mature and continue to reproduce. Each mature female can live up to five years. They lay larvae in the host’s feces, which exit the body and perpetuate their endless life cycle. Sometimes, the larvae do not leave the body through feces; instead, they molt to develop into a parasitic larval form in the intestine. These larvae can then penetrate the intestinal wall and spread throughout the body to the liver and lungs.
The Strongyloides infection is widely prevalent globally. It is estimated that around 613.9 million people are infected, which is equivalent to 8.1% of the global population. Southeast Asia is the region with the highest infection rates, with Myanmar being the country with the highest prevalence in the area.
In Vietnam, statistics from direct stool examinations show that the infection rate of Strongyloides in the community ranges from 0.2% to 2.5%. A study conducted on 2,000 serum samples from patients visiting the Hanoi University of Medicine in 2016 and 2017 found an infection rate of 20%.
Hosts infected with Strongyloides often show symptoms related to the digestive system such as abdominal bloating, abdominal pain, and diarrhea. They can also cause rashes, usually on the legs, and sometimes spread throughout the body. If the infection is severe, it may lead to coughing, wheezing, and symptoms resembling meningitis.

Strongyloides can cause many dangerous complications in the digestive tract. (Photo: Dreamtime).
Additionally, the disease may cause damage to the gastrointestinal mucosa, leading to ulcers or intestinal perforation. Patients may experience pain in the upper right abdomen, diarrhea, irregular fever, and cough. In severe cases, diarrhea may contain mucus and blood, leading to anemia, edema, and ascites.
Treatment: Upon detection of a Strongyloides infection, immediate treatment is necessary. The medications typically used for this type of worm are ivermectin or albendazole. If you suspect an infection, seek medical attention promptly to prevent dangerous complications.
Prevention: The worm is commonly found in tropical and subtropical areas, where direct skin contact with contaminated soil should be avoided. It is also important to establish waste treatment systems.
7. Toxoplasma gondii – Causes Toxoplasmosis
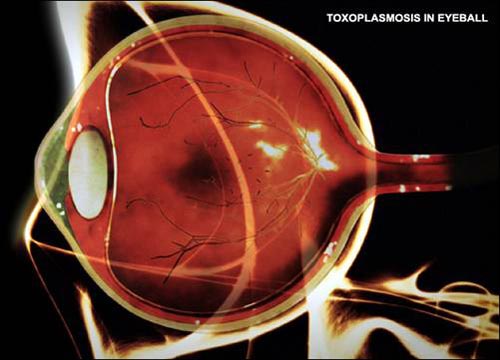
Toxoplasma cysts in the eye
Toxoplasmosis is caused by the single-celled parasite Toxoplasma gondii. Cats are the only hosts during the reproductive stage of this species, making them a common source of infection. The life cycle of this parasite begins when it reproduces a large number of oocysts in the cat’s small intestine. These oocysts are expelled via the cat’s feces. Subsequently, these feces can be ingested by rodents such as mice. Interestingly, when infected with this parasite, rodents often exhibit neurological changes. The parasite effectively takes over the part of the rodents’ brains that controls their fear of cats, causing infected mice to lose their fear and thus become prey for cats, thereby continuing the parasite’s life cycle.
Humans can accidentally become infected with this parasite through ingestion or other transmission routes. Infected cat feces can contaminate household items, soil, and even drinking water. Pregnant women infected with Toxoplasma can transmit the parasite to their fetus.
Once inside the human body, the Toxoplasma parasite forms cysts in tissues, commonly in muscle, brain, and eyes. Though many individuals may be infected, they often show no symptoms due to their immune system controlling the parasite. When the body is ill and the immune system weakens, flu-like symptoms may appear, lasting for a few weeks before disappearing. However, the parasite remains dormant in the body and can reactivate when the immune system weakens, leading to acute eye diseases such as chorioretinitis, which may cause vision loss.
Some other studies are underway to investigate whether the parasite—known for its ability to induce behavioral changes in infected animals—has any connection to schizophrenia.
In cases where pregnant women are infected, it may lead to miscarriage, stillbirth, or congenital toxoplasmosis in the newborn, characterized by unusually large heads.
Treatment: Healthy individuals with resistance to this parasite usually do not require treatment. For severe cases, treatment may involve a combination of pyrimethamine, sulfadiazine, and folinic acid. Pregnant women, newborns, individuals with eye diseases, and those with weakened immune systems may require additional supportive treatments.
Prevention: Eat cooked food and drink boiled water. Freezing meat for several days can also reduce the risk of infection. Wash fruits and vegetables thoroughly, and wear gloves when gardening. Feeding cats cooked food is advisable. Pregnant women should avoid contact with cats.