Research on “jumping genes” has revealed their fundamental role in the aging process.
Scientists continue to explore the secrets of aging to find ways to improve and reverse this natural process, helping us achieve a longer, healthier life. Now, the discovery of jumping genes has shed light on where these secrets lie.

The discovery of “jumping genes” may be the key to achieving eternal youth. (Image: Getty).
Everyone possesses transposable elements (TE) within their DNA sequences, which are sequences that can move or “jump” from one part to another.
If DNA is like a biological blueprint for the body, then TE is a part of this blueprint that can move within the genome. This is a natural process in humans and other animals, but if not carefully controlled, it can cause numerous problems.
Previously, researchers at Eötvös Loránd University in Hungary identified a series of molecular reactions known as the Piwi-piRNA pathway and its role in controlling TEs. They are currently continuing their research to determine whether regulating the Piwi-piRNA pathway alters the aging process in the nematode worm Caenorhabditis elegans.
Molecular geneticist Adam Sturm at Eötvös Loránd University stated that in experiments concerning lifespan, simply adjusting the levels of TE in the Piwi-piRNA pathway resulted in an increased lifespan for the test animals. “This opens the door to countless potential applications in the fields of medicine and biology,” he noted.
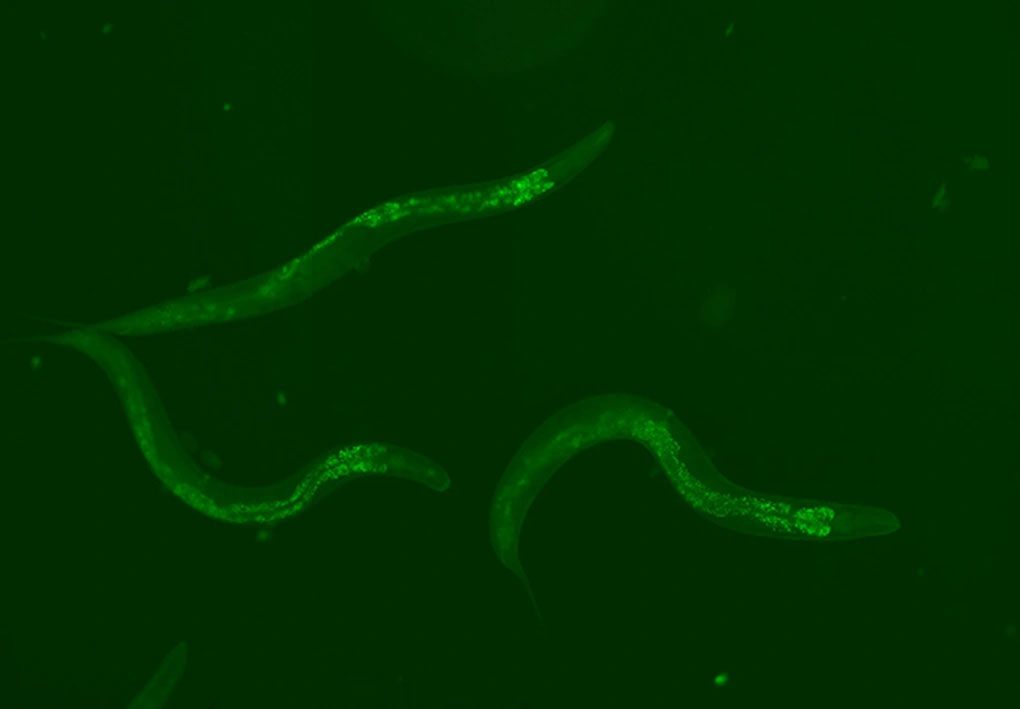
The Piwi-piRNA pathway illuminated within the body of the Caenorhabditis elegans worm. (Image: Nature Communication, 2023).
Experimental results show that worms with reduced TE activity live significantly longer. This indicates that one reason our bodies age is due to how these jumping genes move around within the DNA genome.
This finding aligns with studies on the immortal jellyfish, a marine species capable of continuous regeneration, which theoretically never dies unless diseased or eaten by another species. Researchers have also observed that TEs in this jellyfish are suppressed.
However, it remains unclear whether cellular aging affects TE activity or if TE activity influences cellular aging. With the study on worms, it seems that TEs affect cellular aging, suggesting that other organisms may experience similar effects.
Additionally, researchers noted an increase in N6-adenine DNA methylation within TEs. This change in gene activity enhances TE activity, meaning that as worms age, TE activity intensifies, leading to the aging of the organism.
This is an intriguing discovery because based on these results, we might be able to modify and influence TEs so that cells do not naturally age. Molecular geneticist Tibor Vellai at Eötvös Loránd University mentioned that this epigenetic modification could pave the way for a method of determining age from DNA, acting as an accurate biological clock.
“We may never become immortal like the immortal jellyfish, but we can ensure that the elderly suffer from fewer diseases,” he said.



















































