The superatomic material Re6Se8Cl2 can propel information-carrying particles at speeds twice that of electrons in most current semiconductor materials.
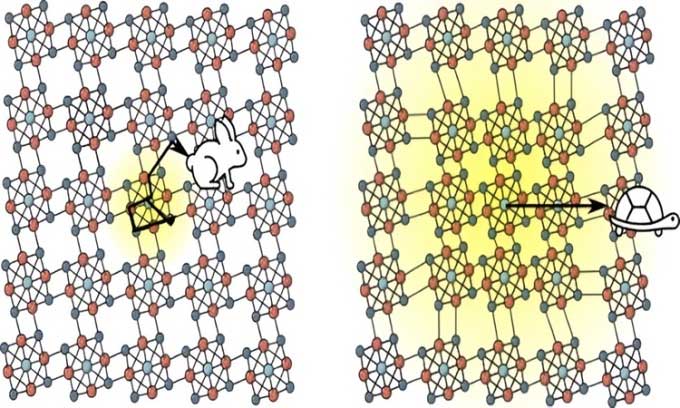
Comparison of silicon structure (left) and Re6Se8Cl2 (right). (Image: Jack Tulyag).
Mainly composed of silicon, semiconductors are present in memory modules, microprocessors, and various types of chips in all electronic devices from smartphones to ovens. However, all semiconductor materials lose energy in the form of heat. A research team at Columbia University has discovered a new superatomic semiconductor that is more efficient than any previously existing products. In experiments, this semiconductor transported quasi-particles at twice the speed of electrons moving through silicon, making it the fastest semiconductor in the world, IFL Science reported on October 30.
Due to atomic structure, all materials vibrate. These vibrations generate quantum particles called phonons. Phonons cause energy-carrying particles in electronic devices to scatter, slowing down the speed of information transmission. However, the superatomic semiconductor known as Re6Se8Cl2 is not affected by this rule. Unlike conventional materials, where energy-carrying particles scatter upon contact with phonons, in Re6Se8Cl2, they bond together. This bonding forms a unique quasi-particle known as the exciton-polarons. They can move without scattering, promising a pathway for faster and more efficient devices. Researchers describe Re6Se8Cl2 in the journal Science.
These quasi-particles not only travel through Re6Se8Cl2 at speeds twice that of electrons in silicon, but they can also traverse large distances. Instead of using electricity, the quasi-particles are controlled by light, meaning theoretically, devices based on such configurations could operate on a femtosecond scale, six times faster than gigahertz chips. All of this can be achieved at room temperature. “In terms of energy transport, Re6Se8Cl2 emerges as the best semiconductor we have ever known,” commented Professor Milan Delor at Columbia University.
The journey of Re6Se8Cl2 began in the laboratory of Dr. Xavier Roy from the Department of Chemistry at Columbia University, which specializes in creating superatoms. These are clusters of atoms that act as a single entity with characteristics different from the constituent elements. Re6Se8Cl2 is composed of rhenium (Re), selenium (Se), and chlorine (Cl). When Jack Tulyag, a Ph.D. student in Milan’s lab, first introduced it, his goal was not to discover a breakthrough semiconductor. Instead, the primary aim was to test the resolution of a new microscope on the material. However, the results surprised them. Instead of sluggish movement, they witnessed motion at unprecedented speeds.
Despite the remarkable potential of Re6Se8Cl2, it still has limitations. A key component is the rhenium element, one of the rarest on Earth, making it relatively expensive. The research team is striving to find other superatomic materials that could surpass Re6Se8Cl2, composed of more common chemical elements.