The UK Medicines and Healthcare products Regulatory Agency has approved the world’s first gene-editing drug for the treatment of sickle cell disease.
This decision, announced on November 17, could save thousands of lives in the country. The drug, named Casgevy, is produced by Vertex Pharmaceuticals. It is based on CRISPR gene-editing technology, which earned scientists the Nobel Prize in 2020. The drug is intended for individuals aged 12 and older diagnosed with sickle cell disease and thalassemia.
The decision was made following a study involving 29 sickle cell patients. After using the drug, 28 individuals did not experience serious issues for at least one year of treatment. In a study with 42 thalassemia patients, 39 did not require blood transfusions for at least one year afterward.
Previously, the only long-term treatment method for these two diseases was bone marrow transplantation. However, this is an extremely arduous procedure that comes with many uncomfortable side effects. According to Dr. Helen O’Neill from University College London, the future of life-changing treatments lies in CRISPR gene-editing technology.
Casgevy works by targeting problematic genes in the patient’s bone marrow stem cells, helping the body produce normal functioning hemoglobin.
Initially, patients require chemotherapy. Next, doctors will collect stem cells from their bone marrow and edit the genes in the laboratory. The modified cells are then infused back into the patient for long-term treatment. Patients must be hospitalized at least twice: first to collect the stem cells and second to receive the edited cells.
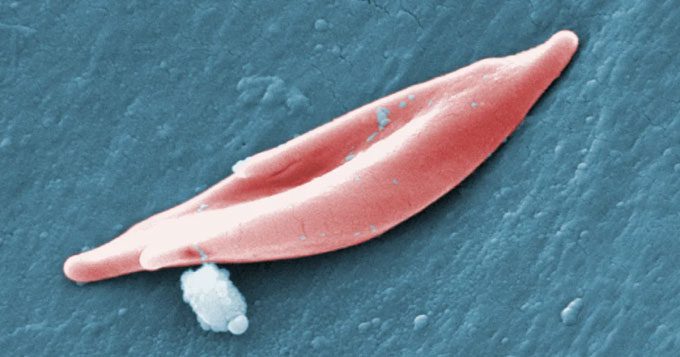
A sickle cell. (Photo: CDC).
Dr. James LaBelle, director of the pediatric cell and stem cell therapy program at the University of Chicago, noted that this could be a new wave of treatment for sickle cell patients. The UK’s approval sets the stage for similar decisions in the United States.
The U.S. Food and Drug Administration is also reviewing this drug and another potential method, with a decision expected in early December.
Sickle cell disease and thalassemia both stem from defects in the gene that carries hemoglobin, the protein in red blood cells that carries oxygen.
In individuals with sickle cell disease, a gene mutation causes the cells to take on a crescent shape, obstructing blood flow and resulting in severe pain, organ damage, strokes, and numerous other complications. The disease is particularly prevalent among individuals of African or Caribbean descent.
For those with thalassemia, the gene mutation leads to severe anemia. Patients require blood transfusions every few weeks, along with lifelong medications. This condition primarily affects individuals of South Asian, Southeast Asian, and Middle Eastern descent.
Last year, the UK approved a treatment for a fatal genetic disorder, priced at £2.8 million ($3.5 million).




















































