Recently, on December 20, the FDA officially approved the world’s first injectable drug to reduce the risk of HIV infection.
The new drug – Apretude serves as an alternative to existing oral preventive medications such as Truvada and Descovy. According to the Centers for Disease Control and Prevention (CDC), oral preventive medications available on the market can reduce the risk of sexually transmitted HIV infection by up to 99%, but they must be taken daily to be effective. In contrast, Apretude requires an initial dose of 2 injections spaced 3 months apart, followed by maintenance injections every 2 months.
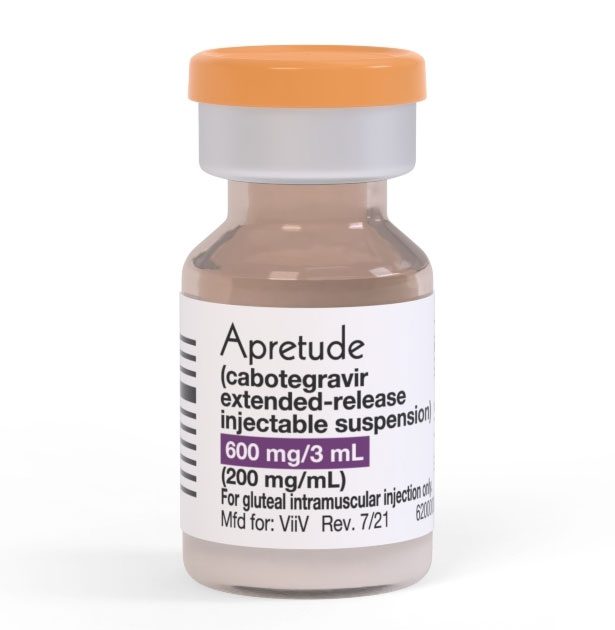
Apretude – the world’s first injectable HIV prevention drug.
“The bi-monthly injections will play a crucial role in addressing the HIV epidemic in the U.S., including support for high-risk groups and certain populations for whom daily medication is not a viable option,” said Dr. Debra Birnkrant, director of the Division of Antiviral Products at the FDA’s Center for Drug Evaluation and Research.
Accordingly, Apretude is approved for adults and adolescents at risk with a minimum body weight of 35 kg. Individuals can choose to take oral cabotegravir, also known as Vocabria, daily for 4 weeks before the injection. This will help assess how well the body tolerates the medication. Additionally, individuals receiving the injection must be tested for HIV and confirmed negative before each injection to avoid the risk of the virus developing drug resistance.
The FDA approved Apretude based on results from two clinical trials showing that this injectable drug effectively reduces the risk of HIV infection more than the daily oral Truvada. The first trial included nearly 4,600 men who have sex with men, and the results indicated that Apretude users had a 69-fold lower risk of HIV infection. The second trial involved approximately 3,200 transgender women at risk of HIV, where Apretude proved more effective, reducing the risk of HIV infection by up to 90%.
However, participants using Apretude experienced more side effects compared to those taking Truvada. Side effects included headaches, fever, fatigue, back pain, muscle pain, rashes, and injection site reactions.
Apretude is priced at approximately $3,700 per dose, totaling $22,200 per year for 6 injections, and is expected to be distributed to retail facilities early next year. In July, the federal government mandated that most insurance companies in the U.S. cover the costs of Truvada and Descovy, as well as laboratory tests. However, to date, insurance companies have not been required to cover the costs of Apretude.