The Covid-19 vaccine from the Beijing Institute of Biological Products (China) is the sixth vaccine to be included in the World Health Organization’s emergency use listing, with a reported efficacy rate of 78.2%.
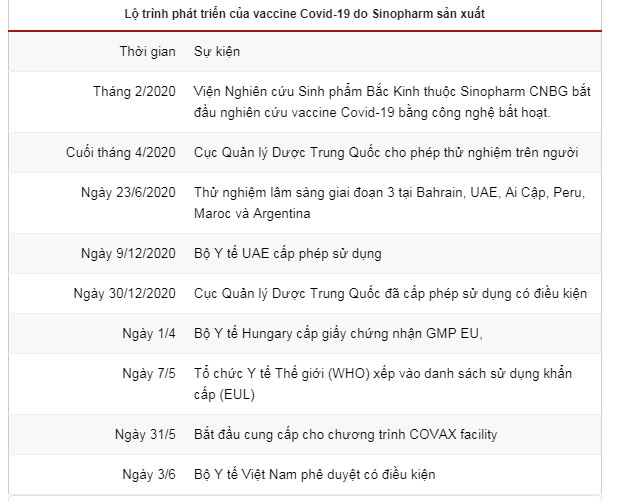
According to information from the Ministry of Health, after the Covid-19 outbreak in China in February 2020, the Beijing Institute of Biological Products, part of Sinopharm CNBG (China National Pharmaceutical Group), began researching the production of a vaccine using inactivated technology named Vero Cell.
At the end of April 2020, this vaccine was approved by the National Medical Products Administration of China for human trials, following successful laboratory studies and animal testing.
A leader from the Ministry of Health stated that this vaccine will be prioritized for three groups of individuals including:
- Chinese nationals working in Vietnam.
- Individuals wishing to study or work in China.
- Residents in border areas.
Covid-19 Vaccine Approved for Emergency Use by WHO
According to the South China Morning Post, starting June 23, 2020, Sinopharm conducted Phase 3 trials of the vaccine with over 40,000 volunteers aged 18 to 59 in Bahrain, the UAE, Egypt, Peru, Morocco, and Argentina.
On December 9, 2020, the UAE Ministry of Health approved the official registration of Sinopharm’s Covid-19 vaccine, announcing an efficacy rate of 86%. This made the UAE the first country in the world to approve Sinopharm’s vaccine.
On December 30, 2020, the National Medical Products Administration of China granted conditional approval for this vaccine, reporting an efficacy rate of 79.34% and a neutralizing antibody rate of 99.52%.
Sinopharm’s Covid-19 vaccine efficacy ranges from 78.2% to 99.52%. (Photo: Reuters).
On May 7, the World Health Organization (WHO) approved Sinopharm’s vaccine for inclusion in the emergency use listing (EUL), recognizing an efficacy rate of 78.2%. This is the sixth vaccine globally to be considered for this list and the first using inactivated technology among them.
On June 3, the Ministry of Health of Vietnam granted conditional approval for Sinopharm’s vaccine. This is the third Covid-19 vaccine approved in Vietnam, following AstraZeneca and Sputnik V.
To date, Sinopharm has produced over 450 million doses of the vaccine, with 100 million doses provided through government aid and commercial sales to businesses.
Sinopharm’s vaccine is stored under conditions of 2-8 degrees Celsius, with a shelf life of two years.
Mechanism of Action
According to the New York Times, to create the vaccine, researchers at the Beijing Institute of Biological Products collected three strains of the novel coronavirus from Chinese patients. These strains were then cultured in monkey kidney cells in a bioreactor to select the strain with the fastest replication rate.
The viral load was then treated with the chemical compound beta-propiolactone, which inactivates the virus by binding to its genetic material. Once inactivated, the coronavirus cannot reproduce or replicate. However, the characteristic proteins, including the spike protein that helps the virus attach to human mucosal cells, remain intact.
Vero Cell uses inactivated vaccine technology. (Photo: SCMP).
Scientists mix the inactivated virus with an adjuvant compound to stimulate an immune response to the vaccine. When introduced into the body, the inactivated coronavirus cannot cause disease and is engulfed by antigen-presenting cells of the immune system.
These cells break down the coronavirus into fragments and “report the intruder.” T cells are activated, detecting the small pieces of the intruder and eliminating them. It then remembers the virus to mobilize other immune cells to attack the intruder, responding to the vaccine.
Simultaneously, B immune cells encounter the inactivated coronavirus. The proteins on the surface of B cells come in various shapes; the compatible protein will bind to the virus and “tag” the identifying features of the intruder. T cells attach to this fragment, stimulating B cells to release antibodies.
This entire process is viewed as a lesson, teaching the immune system to respond to live coronavirus. B cells create antibodies targeting the virus proteins, preventing the pathogen from invading other cells.