Simply put, the common substances around us are all positively charged, and these substances are made up of atoms. An atom consists of a nucleus (composed of protons and neutrons) and electrons that orbit the nucleus.
Each proton in the nucleus carries one unit of positive charge, while neutrons have no charge; the number of electrons orbiting the nucleus corresponds to the number of protons in the nucleus, meaning there is one electron for each proton, and each electron carries one unit of negative charge.
In this way, the positive charge of the nucleus and the negative charge of the electrons cancel each other out, resulting in the atom existing in a neutral state.
If an atom or group of atoms donates or accepts one or more electrons, they form charged particles known as ions. Ions that lose electrons become positively charged, referred to as positive ions, while ions that gain extra electrons become negatively charged.
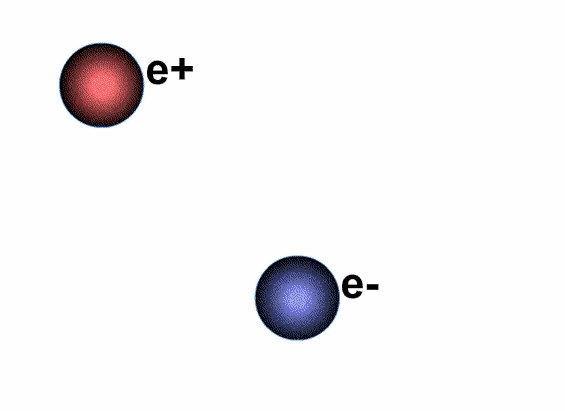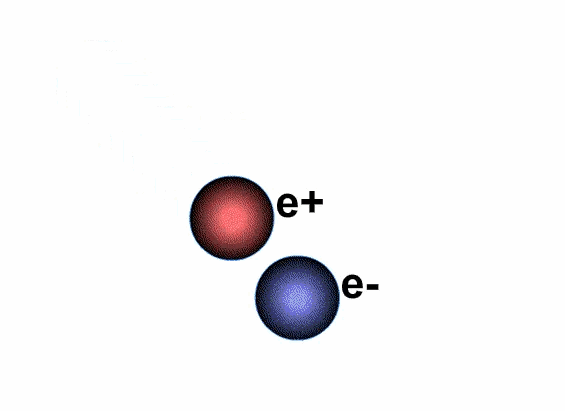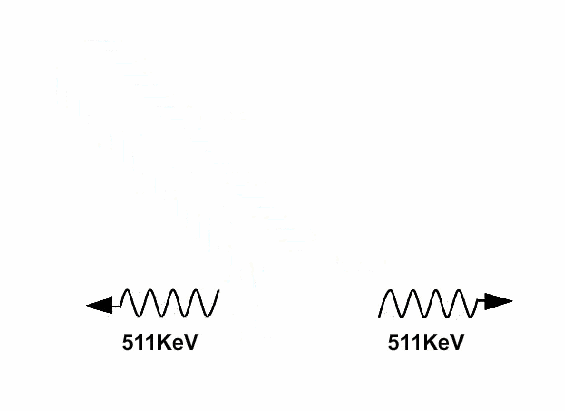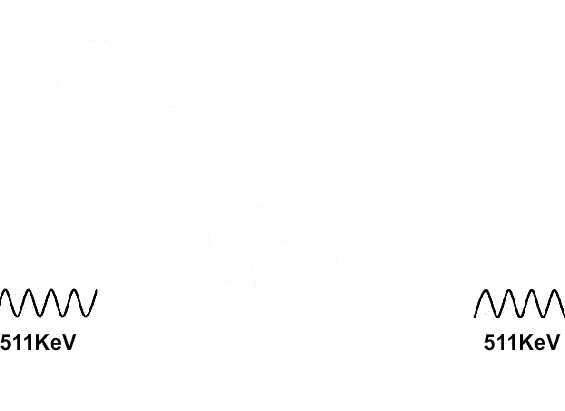
Does antimatter really exist?
Antimatter also consists of atoms, but the charges of the nucleus and the electrons outside the nucleus are completely opposite to the positive charges of matter, meaning the nucleus carries a negative charge while the electrons outside the nucleus carry a positive charge.
Antimatter and positive matter exist independently; if they do not encounter each other, nothing happens. However, if they meet, they will annihilate each other, releasing a tremendous amount of energy.
Nobel Prize-winning physicist Paul Dirac predicted as early as 1928 that every particle must have a corresponding antiparticle, which has the same mass but opposite electric properties. Their spin quantum numbers are also opposite. Therefore, Dirac is regarded as the first scientist to propose the concept of antimatter.
Subsequent scientific discoveries have confirmed Dirac’s predictions, and antimatter exists widely in the universe, gathering in significant amounts in certain special regions. In the natural world around us, antimatter also exists in abundance. For instance, potassium-40 in the human body, an unstable isotope, releases positrons.
Bananas also contain a small amount of potassium-40; a banana releases one positron approximately every 75 minutes, which amounts to about 19 positrons per day.
A banana releases one positron approximately every 75 minutes.
As soon as these positrons are produced, they annihilate with the negative electrons in the vast amounts of positive matter in our world, which is why this “antimatter” cannot generate any detectable waves.
In fact, there are 2.6875×10^19 molecules (about 2.7 billion) in 1 cubic centimeter of air. The main gas molecules in the air are oxygen and nitrogen. Each oxygen molecule has 16 negative electrons, while each nitrogen molecule has 14 negative electrons.
In 1995, CERN produced the world’s first batch of antihydrogen atoms in the laboratory; since then, many laboratories around the world have created various antiparticles, such as negative protons and positrons; in 2011, scientists from China and the United States jointly produced antihydrogen-4.
This demonstrates that antimatter truly exists in our world and can be produced artificially. However, to date, the antimatter discovered and created by humans remains at the atomic or subatomic level, and a whole piece of antimatter visible to the human eye has yet to be discovered.

Antimatter is theoretically the most explosive material, capable of releasing tremendous energy.
How much energy does antimatter possess?
So far, antimatter is considered the material with the potential for the greatest explosive energy, and it can only be converted according to Einstein’s mass-energy equation: E=MC^2. Here, E is energy, measured in joules (J); M is the mass of the matter, measured in kilograms (kg); and C is the speed of light in a vacuum, measured in meters.
Einstein’s mass-energy equation is one of humanity’s greatest discoveries of the 20th century. Although this formula is very simple, it explains a profound truth: mass and energy can be converted into each other and are equivalent.
Converting mass into energy is not a simple task; the most advanced technology humans currently possess for converting mass into energy involves nuclear fission and nuclear fusion. The conversion rate of mass to energy in nuclear fission is about 0.13%, while the conversion rate in nuclear fusion is approximately 0.7%.
The annihilation of antimatter upon contact with positive matter occurs instantaneously.
This conversion rate may sound small, but in reality, it can release energy on a staggering scale, as seen in nuclear bomb explosions, perfectly validating that the mass-energy equation is extremely accurate.
The annihilation of antimatter when it encounters positive matter occurs instantaneously, transforming 100% of the mass of any material into energy, thus referred to as perfect conversion between mass and energy. Moreover, 1 gram of antimatter must be completely annihilated by 1 gram of positive matter, meaning that the results of the annihilation of a portion of antimatter can yield energy equivalent to double the mass.
According to Einstein’s mass-energy equation, 1 kg of antimatter and 1 kg of positive matter can produce 1.8×10^17 J of energy when they collide.
How much energy is that? It is equivalent to 50 billion kilowatt-hours of electricity, or about 43.02 million tons of explosive energy. This is the power of over 3,300 atomic bombs dropped on Hiroshima simultaneously.
From this, we can conclude that the destructive energy of 1 gram of antimatter and 1 gram of positive matter is about 50 million kilowatt-hours, or the explosive power of 3.3 atomic bombs in Hiroshima.

The cost of antimatter is 62.5 trillion USD/gram.
How expensive is artificial antimatter?
It can be said that there is no material in the world more expensive than antimatter. This is because, to date, all the antimatter created by scientists worldwide over decades remains at the atomic level and is nearly invisible under an electron microscope, which the human eye cannot see.
Fermilab has used trillion-electron-volt accelerators, consuming vast scientific and technological resources, and after 26 years, the total amount of antiprotons produced is only 15 nanograms, or 1.5 parts in 100 million of a gram.
All types of antihydrogen atoms, antiprotons, and other antimatter produced in laboratories worldwide do not exceed 20 nanograms.
The cost of producing antimatter is enormous because a large number of scientists have devoted resources over decades to create very few antiparticles. Just considering the energy consumption for producing antimatter shows that it is extremely costly.
Some experts have calculated that based on the current energy consumption to produce antimatter, it requires 2.5×10^16 kilowatt-hours (kWh) to produce 1 gram of antimatter. Approximately one billionth of a gram of antiparticles is produced each year at a cost of 80 million USD. Therefore, its price stands at an astounding 62.5 trillion USD/gram.
Thus, nothing in the world can be more expensive than antimatter.