Researchers at Duke University (USA) have captured the first real-time 3D footage of a virus in motion, just before it attacks a cell.
While reviewing the two-and-a-half-minute clip for her doctoral thesis, Courtney CJ Johnson (Duke University) felt as if she was watching footage of an intruder attempting to break into a house.
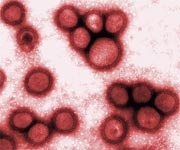
In reality, the clip shows a virus particle, which is a thousand times smaller than a grain of sand, moving and weaving through tightly packed intestinal cells. In a fleeting moment, the virus makes contact with a cell and glides along its surface but does not attach before pulling away.
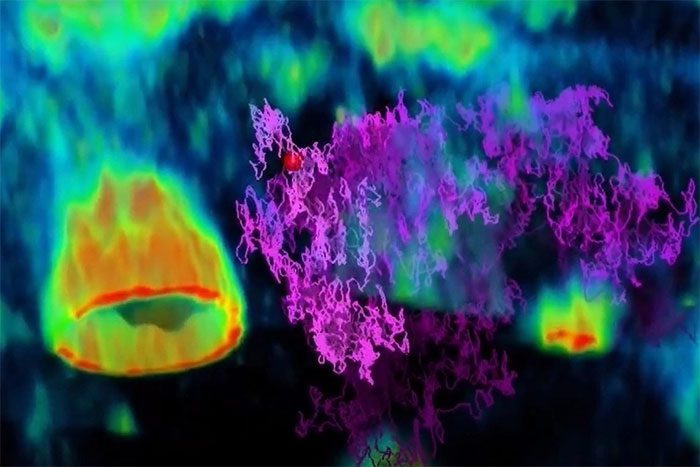
A microscopic video shows a type of virus (purple trace) as it navigates to the surface of human intestinal cells (green). (Photo: Welsher Lab, Duke University).
Johnson is a member of the research team from Duke University (USA) led by Kevin Welsher, an assistant professor of chemistry. She collaborated with PhD student Jack Exell and colleagues to find a way to capture 3D footage of the virus approaching a cell.
We breathe, eat, and encounter millions of viruses every day. Most of them are harmless, but some, such as the influenza virus or COVID-19, can make us sick. The infection process begins when a virus binds to and penetrates a cell, where it takes control of the cellular machinery to produce copies.
“But before it can invade, the virus must reach the cell first,” Johnson stated.
This means the virus has to pass through the protective layer of the cell, the mucus lining the respiratory and intestinal tracts—one of the body’s first defenses against infection. The researchers aim to understand how viruses breach this defensive barrier.
“Tracking the moment before infection begins is inherently difficult, if not impossible, with existing microscopic methods,” Welsher noted.
Part of the reason is that viruses move 2-3 times faster in the space outside the cell. This speed complicates everything, from the imaging perspective to the fact that viruses are hundreds of times smaller than the cells they infect.
Johnson remarked: “That’s why this issue is challenging to study. Under a microscope, it’s like trying to photograph a person standing in front of a skyscraper. You can’t capture the entire skyscraper while still seeing the details of that person in one photo.”
Thus, the team developed a new method called 3D Tracking and Imaging Microscopy (3D-TrIm). It combines two microscopes into one.
The first microscope will “lock onto” the fast-moving virus, scanning the laser around the virus tens of thousands of times per second to calculate and update its position. As the virus bounces and moves around outside the cell, the microscope remains focused on the virus.
While the first microscope tracks the virus, the second microscope captures 3D images of the surrounding cells. Welsher explained that this combined effect is akin to navigating with Google Maps. It not only shows your current location while driving but also the terrain, landmarks, and overall layout of the area, but in 3D form.
With this method, researchers can not only observe a healthy individual inhaling virus particles from the sneezes or coughs of an infected person. First, they must attach a special fluorescent label to the virus before tracking it—what the tracking microscope observes is the movement of the glowing point. Currently, they can only track the virus for a few minutes before it fades.
Jack Exell stated that the biggest challenge now is to create brighter viruses. Additionally, Welsher hopes this technique will aid in tracking viruses that are active outside of coverage and within real tissue—where infections first occur.