Researchers Reveal How Chemical Combinations Created the Acid Fog that Killed 12,000 in 1952.
Imagine a day when you can’t see your own feet, the sunlight is completely obscured, and breathing becomes difficult. This was the nightmare that occurred on December 5, 1952, when a mysterious fog enveloped the entire city of London.
On December 5, 1952, as the capital London was bustling with shoppers preparing for Christmas and the New Year, a thick smog appeared, reducing visibility to just 10 meters.
In the Isle of Dogs area, the fog was so dense that people could not see their own feet. The smog had a horrible smell, with some describing it as resembling rotten eggs. Reports indicated that the toxic smoke was so polluted that it caused livestock to suffocate in the fields.
People did not pay much attention, thinking it was just bad weather and that the foul smell would pass quickly. But that was not the case; on the morning of December 6, London awoke to a bright blue sky, yet the fog was even thicker, and the smell was worse. By December 7, the smog had intensified, and the air became heavier, making it hard to breathe outdoors. Those with lung issues had to go to hospitals due to difficulty breathing or prolonged coughing fits.
As the fog gradually dissipated, thousands of people were found to have died. It is estimated that there were around 4,000 fatalities at that time.

The “killer” fog that enveloped London in 1952.
The secret behind the origin of that deadly fog has long puzzled researchers. Recently, the solution has been unveiled.
In a new analysis, researchers have accurately identified the chemical processes combined with natural fog resulting from coal burning… that created a deadly acid cloud.
Specifically, it was the sulfuric acid particles mixed with natural fog that were the “silent killers” in London at that time.
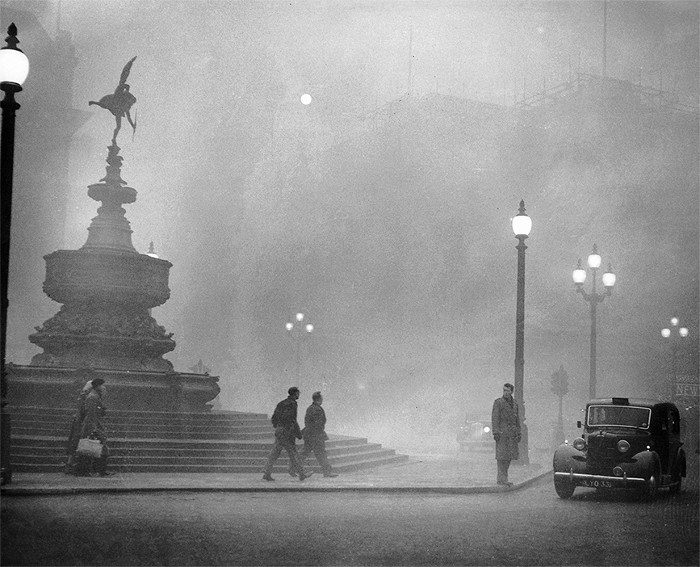
Thick fog obscured the sunlight, causing thousands to experience respiratory issues.
Back in 1952, when the first fogs appeared, the people of London were not informed, and then a few days later, as the fog thickened and obscured the sunlight, thousands experienced respiratory problems.
As a result, at least 4,000 people died, with over 15,000 others hospitalized, and thousands of livestock also perished. According to some experts, the estimated death toll could reach up to 12,000 people.

The death toll from the “killer fog” in London could reach 12,000 people.
According to Professor Renyi Zhang and Harold J from the University of Texas, sulfuric acid particles were formed from sulfur dioxide found in coal combustion and emissions from power plants as well as various vehicles.
The combined effect of sulfur gases in the fog and dust in the air formed this dense smog. The dust primarily originated from coal smoke particles, while components such as sulfur gases in the air, silicon oxides, and aluminum oxides could form droplets, catalyzing sulfur gases in the air, leading to oxidation reactions forming SO3, resulting in “sulfuric acid fog” hazardous to human health.
A large amount of toxic gas and dust in the sulfuric acid fog, when inhaled, would adhere to lung cells, gradually accumulate, and enter the bloodstream, spreading throughout the body.
Experts state that such deadly fogs are becoming increasingly common in modern countries like China. However, the pollution issues are not entirely the same.

These deadly fogs are becoming increasingly common in modern countries like China.
In China, sulfur dioxide and nitrogen dioxide are primarily emitted from power plants, vehicles, and ammonia from fertilizer use. While the London fog had a high acidity, the smog in China is generally more neutral but still poses significant health risks.
Currently, experts are striving to call on everyone to join hands in protecting the environment and reducing emissions because if we continue to “release waste” carelessly, the killer fog will reappear and disturb the entire world.