The lightest magnesium isotope in the world has been created, providing new insights into the binding energy of electrons as they orbit around the nucleus.
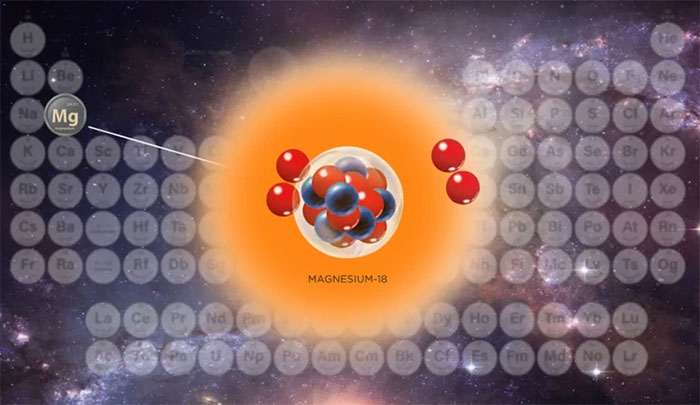
This isotope is called magnesium-18.
Under normal conditions, pure magnesium is a soft, gray metal with an atomic number of 12. This indicates that it has 12 protons—positively charged particles—in its nucleus.
However, recently, scientists have successfully created the lightest magnesium isotope in the world, which contains only 6 neutrons in its atomic nucleus. This isotope is known as magnesium-18.
Although it decays too quickly for direct measurement, researchers hope their discovery will enhance understanding of how atoms are structured.
Thanks to such unusual isotopes, there may be more versions of a chemical element, with varying numbers of neutrons in the nucleus. This helps define the limits of the models that scientists use to understand how atoms function.
According to the research team, the products resulting from the radioactive decay of this isotope provide new insights into the binding energy of electrons orbiting the nucleus. “We are measuring what we can measure to predict what we cannot,” said Kyle Brown, a chemist at the Rare Isotope Research Facility at Michigan State University.
To date, scientists have identified several thousand isotopes of the 118 common elements in the periodic table, with new isotopes being discovered every year.


















































