Scientists have achieved the unimaginable by transforming pure water into a metal and observing this process with the naked eye.
Pure water is an almost perfect insulator because it does not contain free dissolved ions, unlike natural water. In contrast, metals are characterized by their ability to conduct electricity.

The shimmering golden hue on the surface of the metalized water. (Photo: Nature).
However, researchers have achieved the unprecedented feat of turning pure water into a “metal” with conductive properties, observable by the naked eye.
It is worth noting that previously, pure water could only conduct electricity if it was fixed under extremely high pressure. However, this was beyond the current capability of humans to replicate in a laboratory setting.
In this new study, this assumption has been disproven. By exposing pure water to a mixture of alkali metals with electrons—in this case, a alloy of sodium and potassium—the charged particles moved freely, allowing pure water to exhibit fundamental metallic properties for the first time.
Although the conductivity lasted only a few seconds, this marks an important step for researchers to continue exploring the limitless potential of water.
“You can see the phase transition from water to metal with the naked eye,” said physicist Robert Seidel. “The silver sodium-potassium droplet has cloaked itself in a golden hue. That is incredibly impressive.”
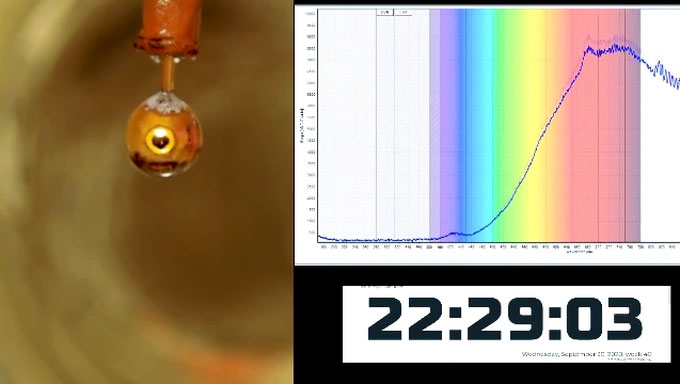
The silver sodium-potassium droplet has cloaked itself in a golden hue, indicating its conductivity. (Photo: Science).
Under sufficiently high pressure, theoretically, any material can conduct electricity.
This occurs when their atoms are pressed tightly together, causing the electron orbitals to overlap and move around.
For water, this pressure limit is approximately 48 megabars, equivalent to 48 million times the atmospheric pressure on Earth at sea level.
In addition to enhancing our understanding of this phase transition on Earth, the research also allows for deeper exploration of extreme pressure conditions on other planets.
For instance, this explains why Neptune and Uranus are believed to easily generate liquid metallic hydrogen through diffusion. Meanwhile, the environment on Jupiter has sufficient pressure to easily transform pure water into a metallic state.
This research has been published in the journal Nature.


















































