For decades, scientists have been striving to synthesize a new allotrope of carbon known as graphyne, and only now have they succeeded.
Graphyne has long attracted the attention of researchers due to its similarities with the “wonder material” graphene—a highly regarded allotrope of carbon that was awarded the Nobel Prize in Physics in 2010. However, despite decades of research, they have been unable to create this material.
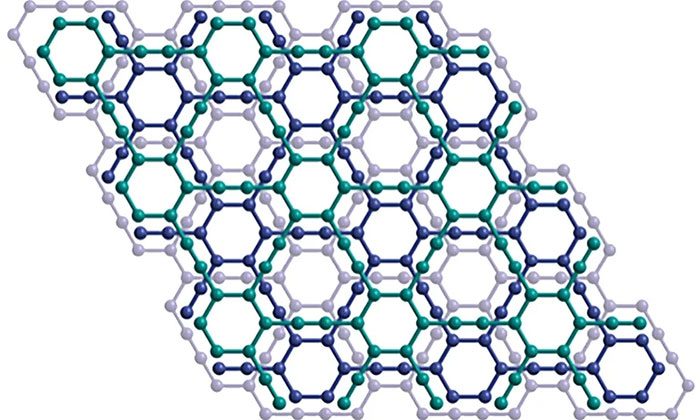
Crystal structure of a layer of graphyne graphene.
Now, that effort has come to fruition, thanks to new research from the University of Colorado Boulder, where they have officially produced graphyne—a material expected to revolutionize the energy and electronics sectors.
Dr. Yiming Hu, the lead author of the study, expressed his excitement, stating, “All of us are incredibly thrilled to have solved this long-standing problem. It has finally been realized.”
Allotropes of carbon can be constructed in various ways depending on how the molecules hybridize, denoted as sp2, sp3, etc. Among these, the most famous are graphite (used in tools like pencils and batteries) and diamond, which are formed from sp2 and sp3 hybridized carbon, respectively.
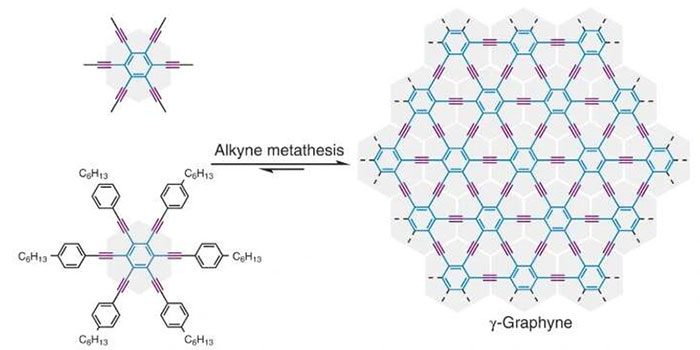
Graphyne forms benzene rings, spaced further apart and connected by Alkyn bonds.
However, at least six decades ago, scientists invented a new format called graphyne. Interest in this material intensified after successfully creating graphene, which has been dubbed a “wonder material” since its invention 17 years ago.
By definition, graphyne resembles graphene even in name. Both are the densest carbon structures ever recorded. However, while graphene has a simple honeycomb structure formed from endlessly repeating hexagonal rings, graphyne is significantly more complex.
Specifically, graphyne forms benzene rings that are spaced further apart and connected by Alkyn bonds. In this structure, every two carbon atoms create a triple bond between three pairs of electrons. “This could be the next-generation wonder material following graphene,” stated Wei Zhang, a chemistry professor at the University of Colorado Boulder.
Although the new material has been successfully synthesized, the research team still aims to further investigate its specific properties to scale up production or craft it into forms useful for everyday life.
The study, published on May 9 in the journal Nature Synthesis, has filled a long-standing gap in carbon material science. Scientists are confident that this research could also open entirely new possibilities for the fields of electronics, optics, and semiconductor materials.