One of the most absurd yet highly entertaining tropes in Hollywood films is the car chase scene. Viewers love a fast-paced, dramatic car chase, and almost all cars (except for the main character’s vehicle) explode upon collision.
The Components of a Car Explosion
To understand whether a car can explode and take to the skies upon impact, we first need to properly understand what an explosion is. All the processes around us in daily life can be explained through thermodynamics, a branch of physics that studies the changes in heat and energy within a system.
In thermodynamics, scientists primarily investigate the properties of a system, such as pressure, volume, and temperature, while we comprehend how these properties change in the form of energy and work (the ability of the system to perform a task).
All processes we observe around us, from cooling food in the refrigerator to water flowing from a reservoir, lead to changes in the aforementioned quantities (along with a bit of entropy, which is the universe’s tendency towards chaos).

Thermodynamics is the study of heat, work, and energy. The term thermodynamics has two meanings: the science of heat and heat engines (classical thermodynamics); and the science of systems in equilibrium (equilibrium thermodynamics). Initially, thermodynamics only referred to the first meaning. Later, the pioneering work of Ludwig Boltzmann introduced the second meaning.
From a thermodynamic perspective, an explosion is a very rapid expansion of volume, accompanied by the release of a large amount of energy. For an explosion to occur, we need flammable gases. These gases must be present in a confined space where they can withstand very high pressure. However, as we know, for something to ignite, it needs a plentiful supply of oxygen. Finally, we will need a spark to ignite the flammable gas.
So… can cars explode?
Before we determine whether a large explosion can occur inside a car’s fuel tank or engine, it is essential to understand that for traditional fuel-powered vehicles to operate, there must be numerous small explosions inside the engine resulting from the combustion of the air-fuel mixture to generate work.
So, if there are small explosions inside the car, could there also be larger explosions?
A typical car engine operates under the laws of thermodynamics and can be viewed as a repetitive process consisting of four stages, hence it is called a four-stroke engine – the shaft is moved by the piston moving up and down.
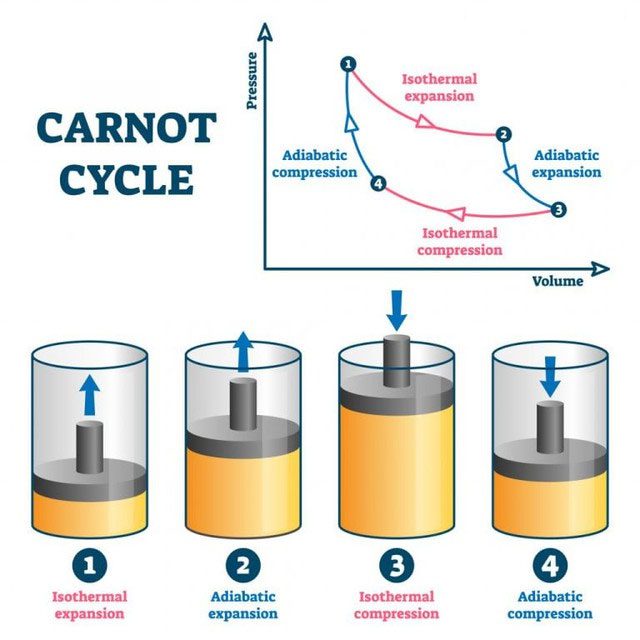
The Carnot cycle principle is used in car engines. Explosions occur to create pressure on the piston, pushing it to move. The cycle of an internal combustion engine in a car consists of four steps to convert gasoline into vehicle movement. This is called the four-stroke engine cycle, which includes intake, compression, combustion, and exhaust.
First, the piston moves down, drawing air into the cylinder (the air-fuel mixture is introduced through open valves). This pushes the piston down. When the valves close, the piston begins to move up, thereby compressing the air in the cylinder.
A small spark creates a very small explosion inside the cylinder (as explained earlier, compressed gas + spark + oxygen = explosion) that pushes the piston down.
Then, the exhaust valve opens, and the air-fuel mixture escapes, causing the piston to move upwards.
These four steps continue to occur rapidly in succession, allowing the vehicle to move forward.

Piston in a car engine.
If there are small explosions, can there be large explosions?
With all that we have just learned, there are certainly things in a car that are flammable and could potentially explode, but are the large explosions seen in movies realistic?
The simple answer is no.
A car contains gasoline, which is a highly flammable liquid, but there are two important points to note about it. Gasoline can be flammable, but it does not exist in an explosive form and is not in gas form inside the vehicle.
Gasoline is introduced into the engine in small amounts as a mixture with air, but not enough to cause a large explosion (only very small amounts are used to push the piston, as shown above). The fuel tank is designed to hold fuel, not to create pressure, so the pressure inside the tank is insufficient to cause an explosion.

One of the favorite “spices” of American action filmmakers or action films in general is explosive scenes, especially vehicle explosions.
Contrary to popular belief (as depicted in films), cars are not easily exploded, and even a burning car is very difficult to explode. The necessary conditions for an explosion are not met after a car experiences a significant collision.
Even setting a car on fire does not cause it to explode; it only burns as long as there is fuel in the vehicle. Therefore, unlike most things portrayed in films, cars exploding and falling apart like shrapnel at the scene is not a realistic outcome!