These findings challenge our understanding of zoonotic diseases and highlight our essential role in the virus exchange process within ecosystems.
The COVID-19 pandemic has brought the transmission of zoonotic diseases into sharp focus. The leading hypothesis is that SARS-CoV-2, the virus responsible for the acute respiratory disease COVID-19, mutated in bats and subsequently infected other wild animals sold live for food, ultimately leading to human infection.
This is not surprising, as we have previously dealt with numerous zoonotic diseases, from Ebola to avian influenza and swine flu. However, the transmission process is, in fact, always a two-way street.
Researchers at University College London (UCL) have now conducted an unprecedented investigation into viral activity, revealing a remarkable truth: humans transmit a greater number of viruses to wild animals than we acquire from them.
Professor Francois Balloux of UCL and co-author of the new study stated: “Human transmission of diseases to animals is far more common than we think. Humans act as the source of these viruses rather than as a mere conduit in the virus exchange between host species.”
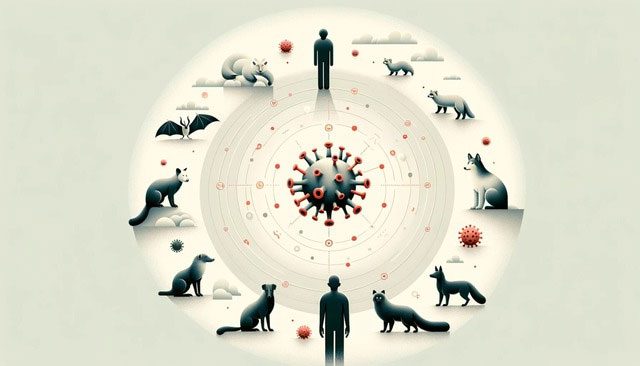
Two-Way Virus Transmission
Anthroponosis, or virus transmission from humans to other animals, is being increasingly recognized. Notable examples include the 2009 H1N1 influenza outbreak in pigs and the SARS-CoV-2 outbreak in minks and white-tailed deer. Pet owners may be more aware of this trend, as dogs, cats, and even ferrets often show flu-like symptoms after close contact with infected humans.
The importance of human-derived viruses has not yet been extensively studied. For their research, the scientists undertook the monumental task of analyzing nearly 12 million viral gene sequences. These sequences were used to construct a phylogenetic tree to help researchers identify patterns of previous host jumps.
Imagine a scenario where a virus is found in both bats and humans, and the viral strains from these two hosts differ only by a few mutations. This similarity suggests a recent transmission event. However, the key question is: who infected whom? This is precisely the purpose of the phylogenetic tree.
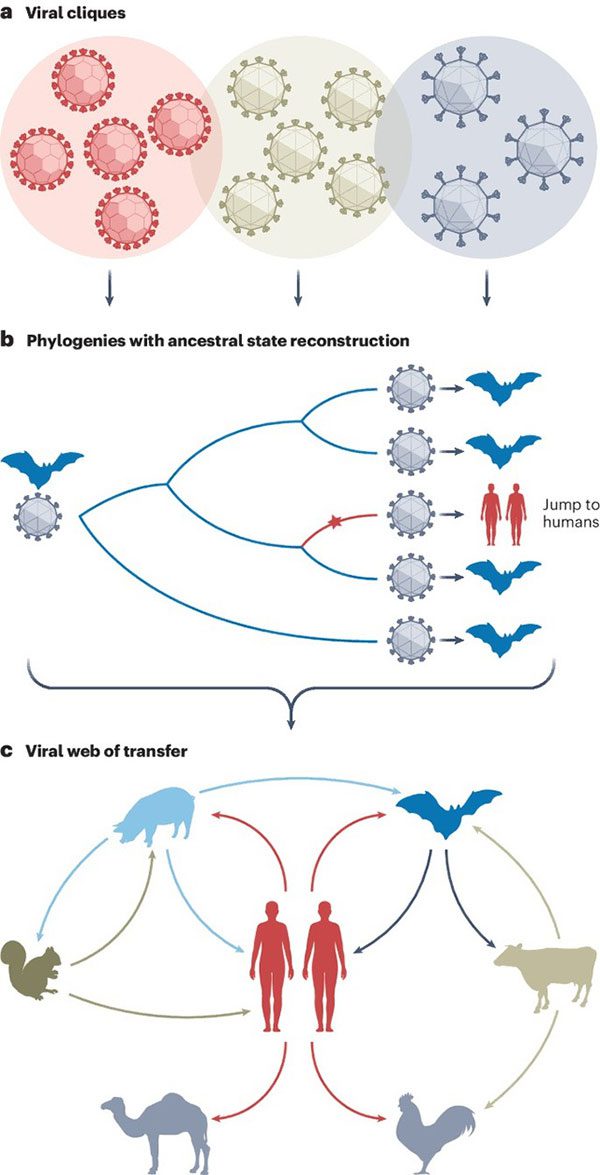
The Viral Family Tree
This classification tree essentially serves as a family tree for viruses. By mapping the evolutionary relationships between different viral strains, scientists can trace their common ancestors. Such trees also allow scientists to infer the direction of viral host jumps.
This is no trivial task, as the sheer volume of data is enormous, and the ecological diversity of viruses is far richer than traditional classification methods can accommodate. To tackle this issue, the researchers constructed their own phylogenetic tree, encompassing 32 families of viruses based solely on genetic relationships.

“In fact, 45% of the sequences lack information about the host, and 37% lack sampling dates. Additionally, we tend to focus on viruses that infect humans, with 93% of all viral sequences related to humans. This significant sampling bias poses a major challenge to existing analytical methods, and we spent a lot of time ensuring our results were not products of this bias,” said Cedric Tan, a researcher at UCL’s Genetics Institute and the Francis Crick Institute, and the lead author of the study, in an interview with ZME Science.
The researchers created a detailed virus classification by grouping related viral sequences and their hosts. They tracked the evolutionary processes of each group to understand how viruses jump between hosts and evolve over time. This work reveals a global viral network infecting vertebrates, providing a new tool for tracking disease spread.
Humans as Key Agents
By meticulously reconstructing the evolutionary history of viruses and examining the genetic mutations acquired during host infections, researchers have completely reversed the notion of humans merely being endpoints of viral diseases. Their most intriguing revelation is that cases of transmission from humans to animals are nearly double those in the opposite direction.
This once again emphasizes the reality that humans are never outside of nature—despite our arrogance that might lead us to think otherwise. Instead, we are an integral part of a complex ecosystem, continuously exchanging pathogens with other species.
The study also explores the genetic basis of these host jumps. They found that viruses often undergo significant mutations as they adapt to new hosts. Interestingly, viruses with a broad range of animal hosts show fewer signs of such adaptive mutations.
“Another interesting finding is that viruses with a wide host range require fewer evolutionary changes to adapt to new hosts. This could explain the common pattern that the most concerning viruses as potential agents of outbreaks and pandemics tend to be capable of infecting multiple hosts,” Balloux noted.


















































