It leaves us a lesson as the Covid-19 pandemic is still ongoing.
Nearly three years have passed since the Covid-19 pandemic first erupted, and the mortality rate from the SARS-CoV-2 virus has dropped to its lowest level since the beginning of the pandemic. This may indicate that the virus is evolving in a way that allows it to spread more easily but causes milder illness.
“There is a common belief that natural forces will resolve this pandemic for us,” said evolutionary biologist Aris Katzourakis from the University of Oxford. Similar to many past epidemics, the virus will either naturally disappear or gradually lose its virulence, becoming endemic and seasonal like the flu.
However, there are exceptions. The evolutionary process of virus strains often has unexpected twists. For virologists, the most illustrative example is the story of Myxoma, a virus that became a nightmare for rabbits in Australia during the 1950s.
Strangely, humans intentionally infected them with the Myxoma virus. The virulence of this strain could lead to a mortality rate as high as 99.8%, and it was used to control the rabbit population that was overrunning the Australian continent.

100 million rabbits were killed by the Myxoma virus in just 6 months.
According to Andrew Read, an evolutionary biologist at Pennsylvania State University, Myxoma killed hundreds of millions of rabbits, making it the deadliest virus known to science for vertebrates. “This is definitely the largest vertebrate massacre caused by a disease,” Dr. Read stated.
The rabbit massacre in Australia continues to this day; even after 72 years, the Myxoma virus still circulates within the rabbit population there. A familiar pattern has been observed: within a few years, the virus’s virulence decreased, and the mortality rate fell from 99.8% to around 50% in the years following the 1950s.
However, in their research, Dr. Read and colleagues discovered that Myxoma reversed its course and increased its virulence again in the 1990s. The latest research published just last June found that the Myxoma virus appears to be evolving to spread more rapidly among the rabbit population.
“It is still throwing out new tricks,” Dr. Read noted.
The Largest Rabbit Extermination in Human History
The story takes us back to 1895 when an Australian farmer named Thomas Austin imported 24 rabbits from England, releasing them on his farm for hunting pleasure.
However, what Austin did not anticipate was that European rabbits, upon arriving in Australia, had no natural predators. There were also no pathogens present that could infect and harm them.
The result was that from just 24 Oryctolagus cuniculus rabbits, their population exploded into millions, consuming all vegetation, invading sheep farms, and threatening the lives of other native wildlife.


Thomas Austin, the one believed to have imported Oryctolagus cuniculus into Australia and caused the rabbit plague.
In the early 1900s, researchers in Brazil proposed a solution for Australia. They discovered the Myxoma virus in a rabbit species native to South America. The Myxoma virus spreads through intermediate vectors like mosquitoes and fleas and is generally harmless to animals.
However, when scientists infected European rabbits with Myxoma in their laboratory, the virus exhibited astonishing virulence. Infected rabbits developed skin nodules filled with the virus.
When the virus infected internal organs, it could kill a rabbit within just a few days. This horrific disease is known as myxomatosis, or rabbit pox. Now, it could become a biological weapon to help Australia eradicate the invasive rabbit population.
Thus, Brazilian scientists transported some of their initial Myxoma virus samples to Australia, where their Australian counterparts spent years testing it in the laboratory.
The goal was to ensure that this viral strain posed a threat only to rabbits and would not affect other animals. Some Australian scientists even self-injected the Myxoma virus into themselves to prove that it could not harm humans.
By 1950, all safety testing of the Myxoma virus in the laboratory had concluded positively. Authorities were finally convinced, and the National Research Council of Australia officially licensed this biological weapon to target the rabbit plague.
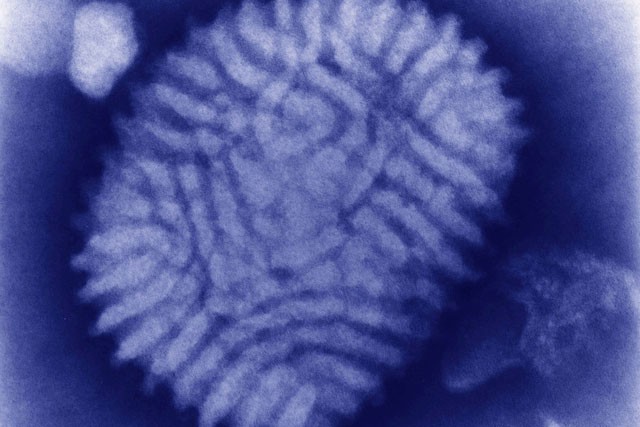
Myxoma virus under an electron microscope.
Scientists sprayed it into rabbit burrows on Wardang Island, located in southern Australia, to see what would happen next. The results were as expected: the rabbits died in droves. Soon, the rabbit pox epidemic spread within a radius of hundreds of miles, and rabbit populations living far from the virus spray site could not escape their grim fate.
Dr. Frank Fenner, an Australian virologist who monitored the rabbit extermination campaign from the beginning, estimated that the Myxoma virus killed at least 100 million rabbits in just six months. Infected rabbits would die in less than two weeks, with a mortality rate of 99.8%. This means that for every 500 infected rabbits, only one survived.
But the virus entered an evolutionary rut
It was thought that Myxoma would spell the end for rabbits in Australia, but evolution once again favored them. After a few years, the few rabbits that survived the pox began to repopulate.
They started to pass on their disease-resistant genes to their offspring. Natural selection continuously identified rabbits that could withstand the Myxoma virus longer and had higher survival rates.
Consequently, the virus’s virulence decreased. By the mid-1950s, Dr. Fenner found that the original Myxoma virus strain had reduced its mortality rate to just 60%. And as it became harder to kill rabbits, it shifted to a strategy of increasing transmission.

Rabbits gathered around a waterhole that had been mixed with the Myxoma virus on Wardang Island.
This development aligned with popular ideas at the time, as many biologists believed that viruses and other parasites would gradually evolve to cause milder diseases. This concept was even encapsulated in a principle known as the law of reduced virulence.
“Long-standing parasites, in their evolutionary process, will gradually have less harmful effects on their hosts than newly emerging parasites,” zoologist Gordon Ball wrote in 1943.
Theoretically, newly acquired parasites can quickly kill their hosts because they have not yet adapted to them. This is disadvantageous for the parasites themselves, as if the host dies too quickly, the parasites do not have enough time to proliferate and infect new hosts. They ultimately perish alongside their host.
Thus, keeping the host alive long enough is also part of the evolutionary strategy of the pathogen itself. This law of reduced virulence also explains why the Myxoma virus living in Brazil is nearly harmless, while when it was first introduced to Australia, it killed rabbits in a frenzy.
Clearly, in South America, Myxoma had more time to evolve and adapt to its hosts.
However, things do not always follow this predictable path. Evolutionary biologists have questioned the logic of the law of reduced virulence in recent decades. Evolving to cause milder diseases may be the best strategy for some pathogens, but it is not the only strategy.

A site releasing the Myxoma virus infecting rabbits in Australia.
“There are pressures that can push the virulence of a pathogen in the opposite direction,” Dr. Katzourakis stated. In 2008, Dr. Read and his colleagues decided to revisit the investigation of the Myxoma virus in their laboratory at the University of Pennsylvania.
“I know this virus was once considered a textbook example,” he said. This means that the attenuation of Myxoma has been acknowledged so evidently that no one doubts it anymore.
Dr. Fenner’s research in the 1950s put the final nail in the coffin for the law of attenuation. “But now, I begin to think, ‘Well, what will happen next?'” Dr. Read said.
Turning Over the Coffin Lid
Fenner completed his final works on the Myxoma virus in the 1960s. And he had good reason to do so. The diary of the renowned virologist indicates that this was the time he shifted his research focus to smallpox in humans.
Dr. Fenner was later appointed as an advisor and eventually became the Chairman of the World Health Organization (WHO) Committee. He was the one who led the world to eradicate smallpox in 1980.
But back to rabbitpox, with Dr. Fenner redirecting his research, the Myxoma viruses were naturally left behind. Thus, in their new study, Dr. Read and his colleagues wanted to revisit Dr. Fenner’s collection of samples from the 1960s.
They transferred these samples to the University of Pennsylvania for comparison with more recently emerged Myxoma virus strains. The researchers also sequenced the virus’s DNA—something Dr. Fenner could not do—and conducted infection studies on rabbits in the laboratory.
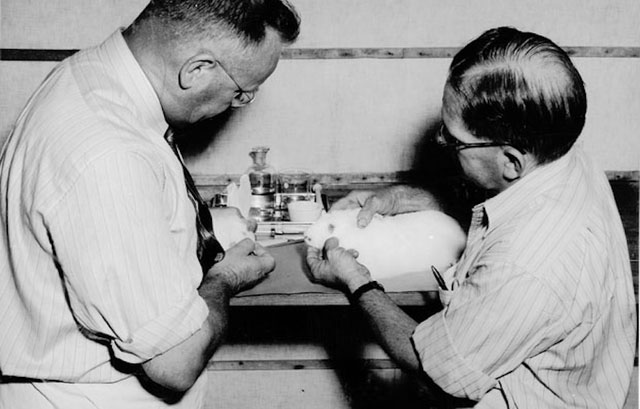
Dr. Frank Fenner, the Australian virologist who pioneered research on the Myxoma virus and rabbitpox.
The results showed that the virus strains dominant in the 1960s caused fewer rabbit deaths compared to the original virus strain. The gene sequencing results continued to confirm Dr. Fenner’s findings. The Myxoma virus had indeed decreased in virulence from 1960 into the 1990s. But then, everything changed.
Newer Myxoma virus strains killed more laboratory rabbits. And even more notably, these viruses did so in a new way. They did not directly kill the rabbits as before; instead, they attacked the host’s immune system.
As the rabbits’ immune systems weakened, they could no longer control the harmless bacteria living in their intestines. These bacteria began to rise as pathogens, causing infections and killing the rabbits.
“The first time we saw that, it was truly frightening,” Dr. Read said.
But strangely, he noticed that wild rabbits in Australia did not share the same gruesome fate as those in his laboratory. Therefore, Dr. Read and his colleagues suspected that the evolution of the Myxoma virus was a response to stronger defenses in the rabbits.
Studies have found that Australian rabbits have acquired new mutations in genes related to their immune system, particularly in the frontline defenses that help them fight disease, known as the innate immune system.

The evolution of the Myxoma virus is a response to stronger defenses in rabbits.
As these rabbits developed stronger innate immunity, natural selection would push back against the Myxoma virus, forcing it to mutate and increase its virulence if it wanted to overcome the rabbits’ defenses.
Both the host and the parasite are now caught in an evolutionary arms race, where the original Myxoma strain briefly outpaced wild rabbits. These viruses even proved to be more aggressive towards rabbits that had not developed innate immunity, such as those in Dr. Read’s laboratory.
And the story is not over; the arms race continues. About a decade ago, a new Myxoma virus strain emerged in southeastern Australia. This strain, known as Strain C, is evolving much faster than other virus strains.
According to Dr. Read’s latest research, the infection experiments he conducted show that with many new mutations, the Myxoma Strain C is performing better at transferring from one host to another.
Many infected rabbits exhibit a strange form of rabbitpox. They develop large swellings on their eyes and ears. These are precisely the areas where mosquitoes prefer to feed—where the virus has a higher chance of reaching new hosts through mosquito bites from intermediate animals.

A rabbit that died from rabbitpox, with swellings filled with virus in its ears.
But ultimately, what can the story of rabbitpox teach us about humans? Virologists see several important lessons that the Myxoma virus can impart as the world grapples with the Covid-19 pandemic.
As the pandemic continues into its third year, we are more protected than ever due to immunity gained from vaccinations or previous infections. However, coronaviruses, like Myxoma, do not necessarily follow a single trajectory of decreasing virulence and causing milder disease.
For instance, the Delta variant last year mutated to cause more deaths than the original version of SARS-CoV-2 in Wuhan. Now, Delta has been supplanted by Omicron, a variant that appears to cause milder illness.
However, virologists at the University of Tokyo have conducted experiments showing that the Omicron variant is evolving into more dangerous forms. “We do not know what the next step in that evolutionary process will be,” Dr. Katzourakis warns. If there were a textbook, then “that chapter in the trajectory of virulence evolution has yet to be written.”