Macroscopic objects are closely linked to their surrounding environment, while microscopic particles can be isolated or protected. This means that the state of macroscopic objects can change due to environmental influences, while the state of microscopic particles may remain unchanged.
Why is there no quantum entanglement in the macroscopic world?
Quantum entanglement is a fascinating physical phenomenon that indicates a connection between two or more microscopic particles that transcends the limits of time and space, allowing them to affect each other even when separated by large distances. This phenomenon is very common in quantum mechanics but rarely observed in the macroscopic world, leading to confusion: since the macroscopic world is composed of microscopic particles, why do the laws governing microscopic particles not apply to macroscopic phenomena?
The macroscopic world is made up of microscopic particles. (Illustrative image)
Many people misunderstand that classical mechanics and quantum mechanics are entirely separate theories with no connection between them. The extraordinary phenomena in the microscopic world simply do not exist in the macroscopic world. However, upon closer examination, it becomes apparent that this perspective is flawed.
Since the macroscopic world also consists of countless microscopic particles, they must be governed by the principles of quantum mechanics. However, classical mechanics is difficult to apply to the motion of microscopic particles. This has been verified throughout the historical development of physics.
In 1924, French physicist Louis de Broglie proposed a bold hypothesis, believing that every microscopic particle has a wave form. This is the famous wave-particle duality hypothesis. With the establishment and development of quantum mechanics, physicists gradually discovered that the particle nature of microscopic particles is merely superficial; their true nature is wave-like.
The wave nature is a fundamental characteristic of microscopic particles. Thus, describing the wave nature of microscopic particles has become a core issue in quantum mechanics. Since particles are waves, these waves have a wave function that will continue to change over time. By describing how the wave function evolves over time, we can capture the motion pattern of these extremely small particles.
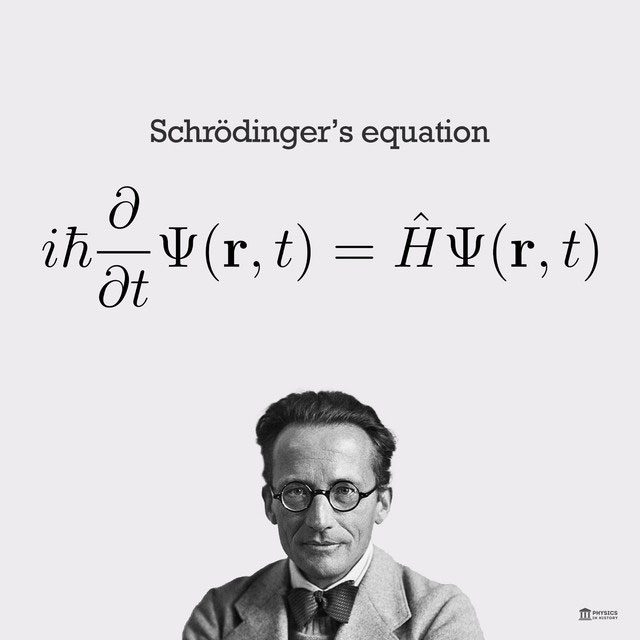
Schrödinger’s equation.
Schrödinger’s equation is a mathematical model used to describe the oscillation of particles over time. However, even with Schrödinger’s equation, quantum mechanics still presents some difficult-to-explain issues. The most famous of these is the wave function collapse problem. Although Schrödinger’s equation can accurately calculate the results of wave-particle collapse, it cannot explain the internal mechanism of wave collapse!
This leads to one of the most mysterious phenomena in quantum mechanics: the uncertainty principle. Anyone familiar with the double-slit interference experiment knows that this experiment is used to verify the wave-particle duality of electrons. If we use an electron gun to emit electrons through two slits at a certain distance, and then detect them on a screen, we will see alternating bright and dark stripes appear on the screen, resembling water waves.
This indicates that electrons exhibit wave-like behavior. However, if we place a detector behind the two slits to determine which slit each electron passes through, the interference patterns disappear, replaced by two cone-like patterns resembling bullets. This shows that electrons exhibit particle-like properties.
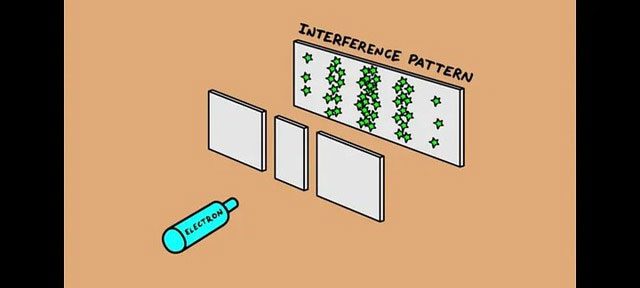
Electrons exhibit both wave and particle properties. (Illustrative image).
Even more astonishing is that if we add a detector behind just one slit and not the other, we will see the interference patterns reappear, but only with half the brightness. This indicates that electrons exhibit both wave and particle properties.
This experiment informs us that the state of an electron depends on whether we observe it or not, and the observable behavior will influence the behavior of the electron. Before observation, the electron does not have a definite position but only a probability of appearing at a specific location.
This is why many scientists believe we should not consider the fluctuations in Schrödinger’s equation merely as a wave; the nature of this wave must be a probability wave, meaning the likelihood of a particle appearing in a certain space is random. Thus, we can boldly imagine that in the wave-particle duality of microscopic particles, if the wave-particle duality resembles a particle more, then the microscopic particles will resemble macroscopic matter more; if it resembles a wave more, then the microscopic particles will resemble a quantum state more.
So why do these quantum phenomena disappear in the macroscopic world? This is due to several significant differences between macroscopic objects and microscopic particles that cause the effects of quantum mechanics to be obscured or eliminated at the macroscopic scale.
Measuring macroscopic objects involves measuring the overall properties of matter. (Illustrative image).
These differences mainly include the following aspects:
- Firstly, the difference in the number of particles. Macroscopic objects are composed of a large number of microscopic particles, while microscopic particles often consist of only a few or are singular. This means that the state of a macroscopic object is a superposition of the states of countless microscopic particles, while the state of a microscopic particle is individual. Therefore, the states of macroscopic objects are more complex and chaotic, whereas the states of microscopic particles are simpler and clearer. This causes the effects of quantum mechanics to be lost or blurred in macroscopic objects.
- Secondly, the difference in environmental interference. Macroscopic objects are closely linked to their surrounding environment, while microscopic particles can be isolated or protected. This means that the state of macroscopic objects will change due to environmental influences, while the state of microscopic particles may remain unchanged. Consequently, the states of macroscopic objects are more susceptible to destruction, leading to the elimination or reduction of quantum mechanical effects in macroscopic objects.
- Finally, there is a difference in measurement methods. Measuring macroscopic objects often uses macroscopic physical quantities, such as mass, position, velocity, temperature, etc., while measuring microscopic particles often uses microscopic physical quantities, such as spin, polarization, energy levels, etc.
This means that measuring macroscopic objects involves measuring the overall properties of matter, while measuring microscopic particles assesses the individual properties of matter. This results in measurements of macroscopic objects being unable to distinguish between different microscopic states and unable to violate Bell’s inequalities, making it impossible to test or prove the effects of quantum mechanics in macroscopic objects.
Quantum entanglement occurs not only with extremely small particles but is a universal physical phenomenon. (Illustrative image).
In fact, in recent years, scientists have experimentally achieved some quantum entanglement at macroscopic or super-microscopic scales, such as the entanglement of two aluminum membranes or two aluminum membranes mentioned in the compelling evidence of quantum entanglement in macroscopic objects.
These experiments demonstrate that quantum entanglement is not limited to extremely small particles but is a universal physical phenomenon that can be observed at any scale as long as certain conditions are met.
In conclusion, we can see that the assertion that there is no quantum entanglement in the macroscopic world is not entirely correct, but it is also not entirely wrong. It reflects the differences and relationships between quantum mechanics and classical physics, while also highlighting many shortcomings and challenges in our understanding of the microscopic world.