Room temperature phosphorescence occurs when a material absorbs energy with a short wavelength (such as UV light) and then emits it as visible light.
This is in contrast to fluorescent materials, which immediately emit light back and stop glowing when the light is turned off.
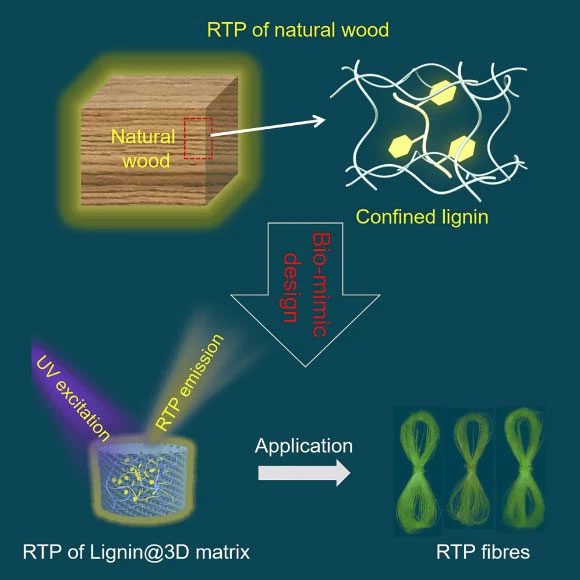
Professor Zhijun Chen, a researcher at the Advanced Wood Materials Engineering Research Center at Northeast Forestry University, and his colleagues discovered that natural Durians and weak phosphors release light within milliseconds due to lignin being retained in the 3D cellulose matrix.
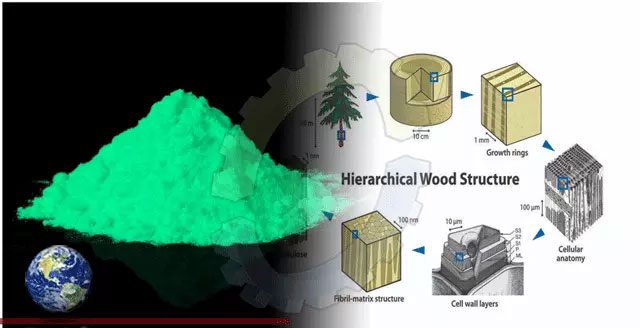
The demand from consumers for renewable, environmentally friendly materials has driven scientists to research wood-based thin films for optical applications. However, until now, these types of materials often have drawbacks, such as poor mechanical properties, uneven light emission, lack of water resistance, or the need to be coated with petroleum-based polymer.
This has inspired them to mimic glowing properties by cross-linking lignin in a 3D polymer network, making it glow more clearly for a longer duration.
“It was truly a surprising and exciting discovery,” Professor Chen said.
“We believe this work will not only provide a new option for sustainable luminescent materials but also a new pathway to fully utilize the values of lignin.”
The researchers found that by adjusting the cavity size in the 3D polymer network and varying the drying time of the polymer, they could alter the persistence time of the phosphorescence.
Phosphorescence, also known as afterglow, is a form of luminescence in which the molecules of the phosphorescent material absorb light, transforming the energy of the photons into energy of electrons in certain quantum states that are high-energy but stable within the molecule. The electrons then slowly drop back to lower energy quantum states, releasing part of the energy back in the form of photons. Phosphorescence differs from fluorescence in that the return of the electrons to their original state, accompanied by the release of photons, is very slow. In fluorescence, the return to the original state of the electrons is almost instantaneous, causing the photons to be released immediately. In contrast, phosphorescence acts like a light storage system: it captures light and slowly releases it later.
“All lignin glows weakly, but most of the light energy is lost due to the vibrations or movements of the lignin molecules, meaning it cannot be clearly seen with the naked eye,” said Professor Tony James, a researcher at the Sustainable Circular Technology Center at the University of Bath.
“We found that fixing lignin in an acrylic polymer allows more energy to be emitted as light – in other words, the less it vibrates, the more it glows.”
“Most current luminescent materials carry toxic components or are very difficult to synthesize, so we aimed to develop a new material that overcomes these limitations.”
“Although this research is still new and has not yet fully explored all aspects, our new material shows great potential in creating a non-toxic, biodegradable phosphorescent material that is more stable and can be used in a wide range of applications.”

Phosphorescent materials are applied to create light sources for situations temporarily lacking illumination but do not require energy consumption to sustain. The stored luminescent energy has been accumulated since the material was naturally illuminated. For example, they can be attached to watch faces to read the time in the dark; affixed to compass needles to determine direction in the dark; or placed on light switches to indicate their location when the lights are off. They are also used for decorations and in the production of luminescent inks (although luminescent inks tend to use fluorescent materials more). Lasers can also utilize phosphorescent materials. This is because electrons can remain in the excited state long enough to wait for other photons to pass through and cause stimulated emission. Phosphorescent materials have also been used in cathode ray screens. After an electron beam strikes a pixel on the screen, that pixel, containing phosphorescent materials, is excited and continues to glow for a short time afterward. However, fluorescent materials can also be used, thanks to the afterimage effect on the retina. Similarly, screens that capture high-energy particle streams (electrons, X-rays, neutrons, etc.) can also contain phosphorescent materials, although fluorescent materials can also be employed.

The origin of the term “phosphorescence” is due to the light emitted in the dark by the phosphorescent phenomenon, similar to the luminescence emitted by phosphor compounds during oxidation chemical reactions in the air. This term has been used to describe materials that glow in the dark without burning, since the German alchemist Hennig Brand discovered phosphorus in 1669 through the distillation of urine. He noticed that the substance he had just distilled glowed in the dark. The term phosphorus itself originates from the Greek word phosphoros, meaning “light bearer.” However, the physical nature of the two phenomena is different; in which the light of phosphorus takes energy from a chemical reaction. The luminescence of phosphorus that Brand observed was actually due to phosphorus burning slowly and smoldering in the air.