The first batch of vaccines will undergo quality testing in Russia within 30 days before both parties sign an official contract.
On July 21, the Russian Direct Investment Fund (RDIF) and Vabiotech LLC officially announced the pilot production of the Sputnik V vaccine in Vietnam. The first batch will be used in the national immunization program after the technology transfer is completed and the quality testing results at the Gamaleya Research Institute (Russia) meet the required standards.

Inside the processing workshop, in the outermost room, staff will perform steps to wash and dry the vials, removing impurities before filling them with the vaccine.

The glass vials must be completely sterile to ensure the quality of the vaccine.

Sterile glass vials will be placed on the conveyor belt, moving to the next room to have the semi-finished vaccine (shipped from Russia) injected into them.

Then, the machine will proceed to seal the vials with rubber caps to ensure the safety of the vaccine.

The vaccine vials move along the conveyor to the aluminum cap sealing machine.

In the adjacent room, unlabeled vaccine vials will be carefully inspected by staff to ensure there are no abnormalities inside.

Once confirmed to be normal, these vaccine vials will be returned to the production line, moving to the next room for labeling by other staff.

Vaccines after labeling will be placed in an emergency freezing refrigerator. Here, the vials will be reduced from -2 to -23 degrees Celsius, meeting the standards of Russia’s special freezing process.
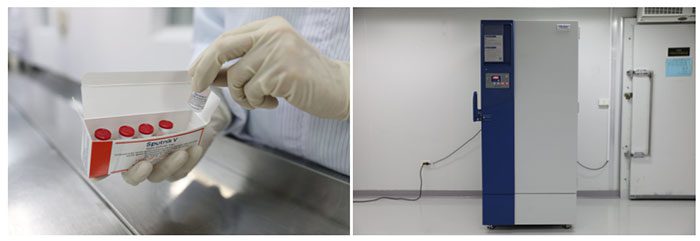
When reaching -23 degrees Celsius, the vaccine vials will be packed by staff and stored in a cabinet at temperatures between 2 to 8 degrees Celsius.

In this pilot batch, Vabiotech processes 30,000 doses. The test samples are currently being sent to the Gamaleya Center (Russia) for quality evaluation. According to Vabiotech, the testing period in Russia will last about 30 days.
After passing quality standards, both parties will negotiate and sign an official processing contract. The contract will also calculate the amount of vaccine Vabiotech retains and the amount returned to the partner. The processing amount could range from 5 to 7 million doses per month, depending on the semi-finished product supplied from Russia.



















































