According to a report published on February 2 by the UK Senate, 9 to 10 types of medication prescribed for infants have not undergone any clinical trials on children.
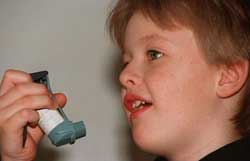 |
Medications used in nebulizers for asthma treatment prescribed for children are typically adjusted based solely on the child’s weight or size to reduce the adult dosage (Photo: TTO) |
Accordingly, pain relief medications such as morphine and paracetamol, as well as drugs used in nebulizers for asthma treatment, are often prescribed for children by doctors based merely on the child’s weight or size to adjust the dosage.
Meanwhile, overdosing on medication is one of the greatest risks identified in many pediatric cases. The report indicates that most pharmaceutical laboratories often overlook auxiliary clinical trials on children due to high costs (approximately 3.6 million euros per product).
This issue is not limited to the UK. According to several documents from the European Medicines Agency (EMA), half of the medications prescribed for children of all ages in Europe have not undergone clinical testing on this demographic.
L.XUÂN (According to AFP)




















































