By replacing the hazardous chemical electrolytes used in conventional batteries with water, scientists have created a type of “water battery” that can be recycled.
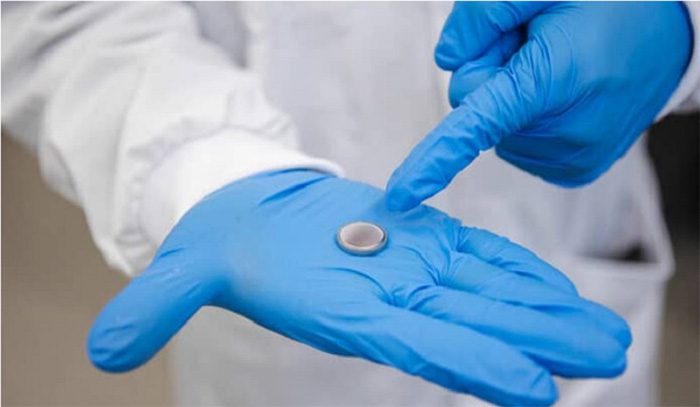
Metal – Ion Water Battery Prototype – (Photo: RMIT University).
The official name of the “water battery” is metal – ion water battery. This type of battery uses metals like magnesium or zinc, which are cheaper and less toxic than the materials currently used in other types of batteries.
In a water battery, the electrolyte is water with a little salt added instead of sulfuric acid or lithium salts.
Notably, the research team has found a way to prevent the short-circuiting phenomenon in water batteries—this occurs when metal fibers known as dendrites appear in the battery—by coating the anode with bismuth metal to inhibit dendrite formation.
The team’s experiments show that this method also helps the prototype water battery operate longer, maintaining over 85% capacity after 500 charge cycles.
According to ScienceAlert on March 5, the team has developed prototypes of water batteries in the size of coins used in watches, as well as cylindrical batteries similar to AA or AAA batteries.
The team leader, Tianyi Ma, a scientist from RMIT University in Melbourne, Australia, stated that while this new technology is unlikely to replace lithium-ion batteries soon, with further research and development, water batteries could become a safe alternative within a decade or more.
Lithium-ion batteries are found in all electronic devices from laptops to smartphones, motorcycles, and e-bikes, and they can overheat and catch fire in some cases.