Despite weighing only about 2% of your body weight, the brain consumes up to 20% of the total energy and oxygen that you take in.
What happens if you stubbornly continue to use a computer or mobile phone that only has a few percent battery left? They will certainly shut down – a phenomenon known as “digital death“, where important data, actions, and tasks you were performing on the device will not be saved.
To prevent this from happening, modern electronic devices are often programmed with a state called “power-saving mode“. This mode is automatically activated when the device’s battery level drops low, below 10%, or can be manually selected by the user.
A smartphone in power-saving mode will turn off some background applications, reduce screen brightness, refresh rate, and even resolution. It has to do this to prioritize energy for more basic activities, at least until the phone is recharged.
Now, in a new study published in the journal Neuron, scientists report that the brain also has a similar energy-saving mode. It is activated when you are hungry until food is ingested, replenishing your energy.
In energy-saving mode, many strange phenomena also occur within the brain, particularly in the cortex that processes signals from the senses. However, people rarely notice them, as these changes often occur so subtly that we take them for granted.
For example, do you know when your visual resolution decreases?

We know the brain is the most energy-consuming organ in the body. Despite only weighing about 2%, the brain uses up to 20% of the total energy and oxygen that you absorb.
Every brain cell requires a stable supply of glucose, which it converts into adenosine triphosphate (ATP), the energy currency that neurons use to process information.
When we first feel hungry, the brain continues to operate at full capacity, consuming the energy allocated to it. However, as humans and other animals have historically faced the risk of starvation for extended periods, sometimes seasonally, scientists believe the brain must also develop “power-saving” mechanisms for such emergencies.
Evidence supporting this hypothesis was recently discovered by neuroscientists at the Nathalie Rochefort laboratory, University of Edinburgh. In their studies, they observed that when mice were deprived of food for several weeks – long enough for them to lose 15%-20% of their body weight – neurons in the visual cortex significantly reduced the amount of ATP used at synapses.
This reduction reached up to 29%, resulting in a cognitive cost: neurons in low-energy mode processed visual signals less accurately, weakening the image resolution that the mice perceived from the outside world.

Upon publication, this work garnered widespread attention and praise from the international neuroscience community. The findings are considered significant for our understanding of how the brain functions, the impact of malnutrition, or even certain diets on our perception of the world.
It also raises the question of whether previous neuroscience experiments conducted on animals yielded accurate results, as scientists designed these studies by starving the animals and not providing them with an optimal energy diet.
This could potentially undermine previous neuroscience research findings. If this is true, it would mark a turning point in the science of brain research.
Initial Evidence of the Brain’s Energy-Saving Mode
If you’ve ever felt that you couldn’t concentrate on work when hungry – or all you could do was sit idly thinking about food – then yes, scientific evidence supports you. It turns out this is a very normal phenomenon.
Many studies in the field of neuroscience have confirmed that short-term hunger can alter neural processing in the brain. It distracts our focus, directing our attention toward searching for food rather than continuing what we were doing.
In 2016, Christian Burgess, a neuroscientist at the University of Michigan, and his colleagues conducted an experiment on mice. They showed two groups of well-fed and hungry mice an image related to food. As a result, a region of the visual cortex in the hungry mice was more stimulated.
After the hungry mice were fed, this stimulation decreased. Similarly, studies on humans have also found that food elicits a stronger response in certain brain areas when volunteers are hungry compared to after they are full.
However, physically speaking, Burgess noted that whether you are hungry or not, “the photons hitting your retina are still exactly the same. The only difference is how they are represented in your brain, because [when hungry] you already have a goal [which is food]. That is something your body knows you need, and it will direct your attention in a way that helps fulfill that need.”

But what happens if you are not just hungry for a few hours, but for a longer time? Researchers realized that at this point, the brain may “downshift.” It reduces activity, shutting down the most energy-consuming processes to enter a “power-saving mode.”
The first evidence of this effect was observed in 2013, in a study on the brains of fruit flies. Two scientists, Pierre-Yves Plaçais and Thomas Preat from the French National Centre for Scientific Research and ESPCI Paris, found that when flies are hungry, they shut down a pathway in their brain. These areas are normally responsible for helping flies form long-term memories, which is an energy-intensive process.
If scientists intervened in the fly’s brain to force this memory pathway to reactivate, the flies would starve much faster compared to when they shut down this process to conserve energy – and perhaps their lives.
However, the next question that scientists posed was: Can larger brains, with higher cognition like those of mammals, including humans, do the same?
They were also unsure whether there is a stronger energy-saving mode that would be activated before animals starve. And there is another paradox: If the energy supply to the brain is cut off too early, it could affect the animal’s ability to recognize and search for food.
All these questions have now been addressed in the new study from the Nathalie Rochefort laboratory. Their paper published in the journal Neuron provides the first insight into the boundary between two processes, as the brain adapts to save energy while ensuring the organism will find the food it needs over the long term.

Specifically, in a three-week experiment, researchers restricted food intake for a group of mice until they lost 15% of their body weight.
Interestingly, they were not starving: In fact, the researchers had fed the mice well right before the experiment began to prevent short-term neurochemical changes associated with hunger that Burgess and other research teams had found. The mice were essentially just not receiving as much energy as they needed.
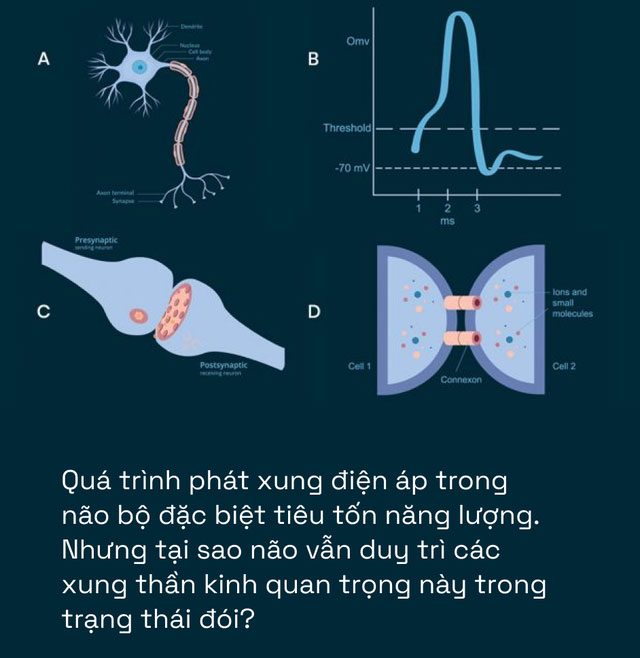
The next task was to eavesdrop on the conversations between the neurons of the mice. Researchers measured the number of action potentials – the electrical signals that neurons use to communicate – sent by a few neurons in the visual cortex. These potentials were measured when the mice were shown an image with black bars oriented at different angles.
Different neurons in the visual cortex react to bars oriented in a preferred direction. For example, if a neuron’s preferred direction is 120 degrees, it will emit action potentials more frequently when the visual stimulus has elements at angles equal to or close to 120 degrees. The rate of these action potentials significantly decreases when the angle is much greater or less than the default square.

Previously, scientists knew that neurons could only emit action potentials when their internal voltage reached a critical threshold – by pumping positively charged sodium ions into the cell.
However, after generating these voltage spikes, neurons must pump all the sodium ions back. In 2001, scientists demonstrated that the sodium pump is one of the most energy-demanding processes in the brain.
Therefore, the authors of the new study targeted this energy-intensive process to find evidence of the brain’s energy-saving tricks. The idea is that the areas where the brain consumes the most energy are also the places most sensitive to hunger.
The results were indeed predictable. When food was scarce, neurons in the mice automatically reduced the current flowing through their membranes—meaning the amount of sodium ions entering—so they wouldn’t have to expend as much energy pumping sodium ions back after generating voltage spikes.
Logically, absorbing less sodium would lead to fewer voltage spikes, but somehow, the hungry mice maintained a similar spike rate to well-fed mice. Therefore, the researchers predicted that some compensatory processes must be occurring to maintain the spike rate in this visual cortex.
Diving deeper, they discovered two changes, both of which help neurons generate voltage spikes more easily. First, the neurons increased their input resistance, reducing the current at their synapses. At the same time, they elevated the resting membrane potential, bringing it closer to the threshold needed to generate a voltage spike.
Anton Arkhipov, a computational neuroscientist at the Allen Institute for Brain Science in Seattle, stated, “It seems that even in a state of hunger, the brain still needs to maintain these voltage spikes for extended periods. Therefore, they must play a crucial role for the organism. Otherwise, the brain could simply turn them off to save a significant amount of energy.”
Of course, maintaining the spike rate must require the brain to sacrifice something. Specifically here, the cells in the visual cortex can no longer selectively choose the angle and axis to which these spikes are transmitted. As a result, their responses become less accurate.
Switching to Low-Resolution Mode
To test whether visual perception was affected by the reduced accuracy of the neurons, the researchers subjected the mice to a new experiment.
They placed them in an underwater chamber with two corridors, each marked by a different image of black bars tilted at a fixed angle against a white background. One of these corridors had a hidden platform that the mouse could use to escape from the water.
The mice were trained beforehand to associate which angle of the black bars corresponded to the side with the hidden platform and escape route. However, by making the angles of the black bars in the two tunnels more similar, the scientists required the mice’s vision to work at a higher resolution to detect them.
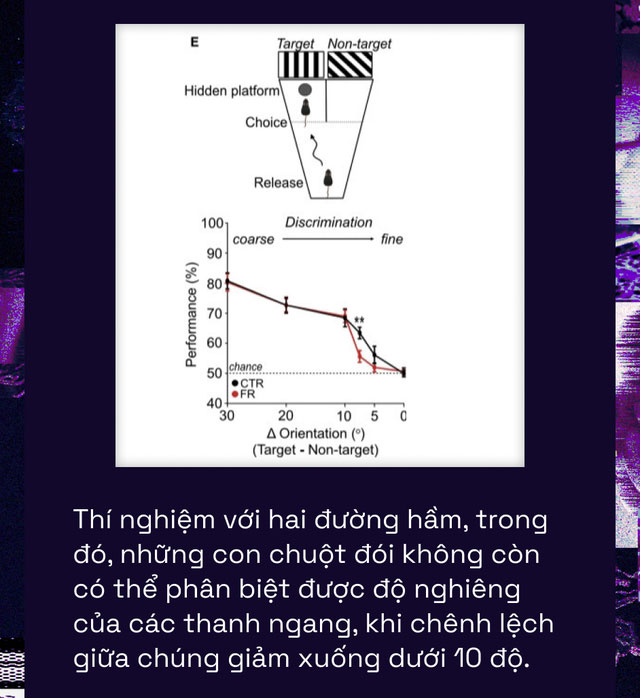
The results showed that when the mice were food-deprived, they could still easily find the exit when the difference between the two images was significant. However, when the difference between the angles of the horizontal bars was less than 10 degrees, suddenly, the mice could no longer distinguish them as accurately as before. They failed to find their way out compared to the well-fed mice.
Thus, the consequence of energy-saving in the brain, while still maintaining voltage spikes, is that the mice must lower the resolution of the world they see to a certain extent.
The brain still prioritizes essential functions for survival when the mice are hungry. Vision must still operate, but it deems a 10-degree difference in the orientation of the horizontal bars unnecessary. Perhaps the mice’s brains determine that this does not affect their ability to find fruit at close range or detect a lurking predator.
Lindsey Glickfeld, a neuroscientist studying vision at Duke University, expressed surprise that these perceptual impairments occurred very early, before the animal entered a state of hunger.
“I was completely taken aback. Somehow, the [visual] system has figured out this way to massively reduce energy use. And it simply involves lowering the resolution of the world, which is a relatively subtle change for animals in their perceptual task performance,” Glickfeld said.

Currently, the research only confirms that mammals can activate energy-saving mechanisms in the neurons of the visual cortex. But as Rochefort and colleagues suspect, it could also occur in other cortical regions.
Many other researchers think so too. Maria Geffen, a neuroscientist at the University of Pennsylvania, stated, “In general, neurons behave very similarly across cortical areas.“
She believes that the energy-saving mechanism may also occur in all other senses during perception when the brain identifies what is most useful for the organism at the moment they are hungry and filters out everything else unnecessary.
“Most of the time, we do not use all our senses to the limits they can reach. Depending on behavioral needs, the brain constantly makes adjustments,” Geffen said.
Fortunately, any blurriness that appears in vision when hungry can be restored, sometimes without the mice needing to refuel. Specifically, researchers in Nathalie Rochefort’s lab reported that they have found a way to turn off the “power-saving mode” or switch back to normal visual functioning in mice.
This is achieved simply by injecting a dose of leptin, a hormone that the body uses to regulate energy balance and hunger sensation. Leptin is now likened to a switch that turns on and off the power-saving mode in the mouse’s brain.
Once reactivated, their neurons responded to the world with high accuracy again. The resolution of vision returned, and the perceptual deficits eventually disappeared.
It is important to emphasize again that these effects occurred without the mice needing to consume any food. “After administering leptin, we were able to trick their brains into restoring cortical function,” Rochefort said.
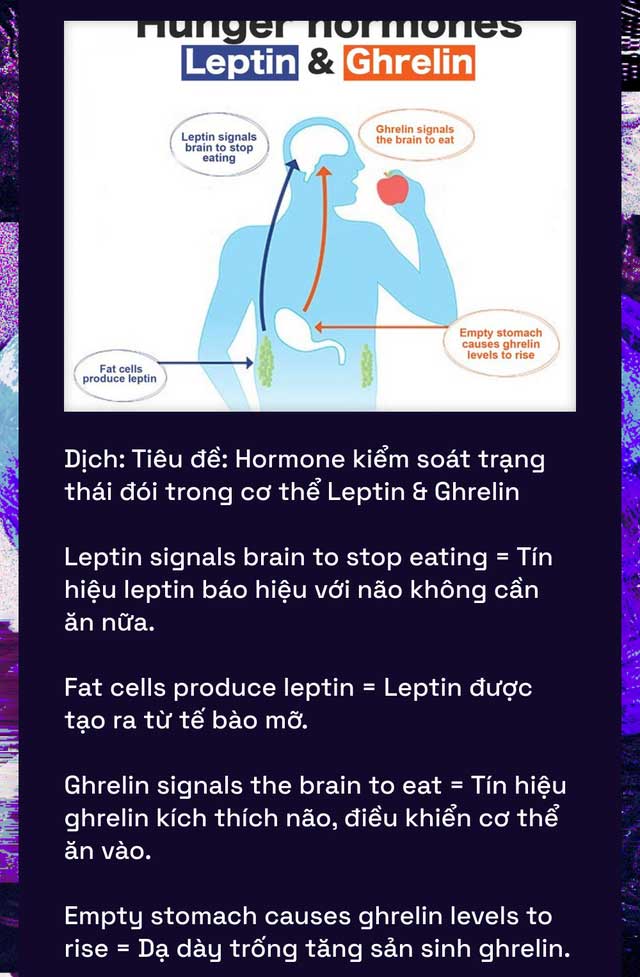
Since leptin is released by fat cells, scientists believe that its presence in the blood can signal to the brain that the animal is in an environment with abundant food and no longer needs to conserve energy.
Conversely, the new study hypothesizes that low leptin levels may alert the brain to a state of malnutrition. And then, it shifts the brain into low-energy mode.
Julia Harris, a neuroscientist at the Francis Crick Institute in London, remarked, “It is rare for a research finding to be as satisfying and fulfilling as these results. It is a beautiful discovery that aligns with our current understanding.”
But could it undermine previous neuroscience research findings?
A quality study is also one that raises more questions than answers. And this is something that some scientists are concerned about.
Much of our current understanding of how the brain and neurons function has been derived from animal studies. In these cases, scientists may have inadvertently placed them into energy-saving mode.
The mice before participating in the experiments are often subjected to fasting or restricted food intake for weeks. This method of inducing hunger is extremely common when scientists want the mice to perform a task in exchange for food rewards.
If they do not starve the mice, they simply want to sit still in their cages and do nothing. If so, it would be like measuring the performance of a smartphone chip while the phone is in “power-saving mode“?
“A truly profound impact of this new research is that it clearly shows that food restriction will affect brain function,” Rochefort stated. She suggested that the observed changes in the flow of charged ions could play a particularly important role in learning and memory processes, as they are based on specific changes occurring right at the synapses.
Glickfeld added, “Now, we need to think carefully about how to design experiments and how we interpret experiments whenever we want to question the sensitivity of perception in animals or the sensitivity of neurons.“
The research results from Nathalie Rochefort’s lab now raise entirely new questions about how various physiological states and hormone signals might affect the brain, and whether hormone levels in the blood can cause individuals to perceive the world a bit differently.
Rune Nguyen Rasmussen, a neuroscientist at the University of Copenhagen, notes that each of us has different levels of leptin and an overall unique metabolic profile. “This means that visual perception may differ from person to person, and within each individual, even though we may not be consciously aware of these differences,” Rasmussen stated.

Rasmussen believes that the conscious visual perception of mice seems to be influenced by food scarcity, as there are changes in the neuronal representation of those perceptions and in the behavior of the animals studied.
However, we cannot know for sure, “because this requires animal species to describe their high-quality visual experiences to us, and clearly, this is something they cannot do,” Rasmussen added.
The next question now is whether the neurons in the visual cortex of other mammal species, including humans, operate similarly. Glickfeld predicts the answer is yes: “Because I think this is actually a relatively basic mechanism of neurons.”



















































