Sometimes, the seemingly simple things in life can leave us puzzled. For example, why does metal always feel colder than wood or plastic when touched, even though both are in the same environment? Or why does a metal baking tray feel hotter than a freshly baked cake?
The answer is actually quite simple but requires a bit of logical thinking: we do not perceive the actual temperature; we only sense how thermal energy moves.
What Is the Sensation of Cold?
Imagine you are standing outside in the cold, facing a tree trunk and a metal lamp post. Even though both are outdoors and at the same temperature, you will definitely feel the metal lamp post is much colder than the tree trunk. The reason?
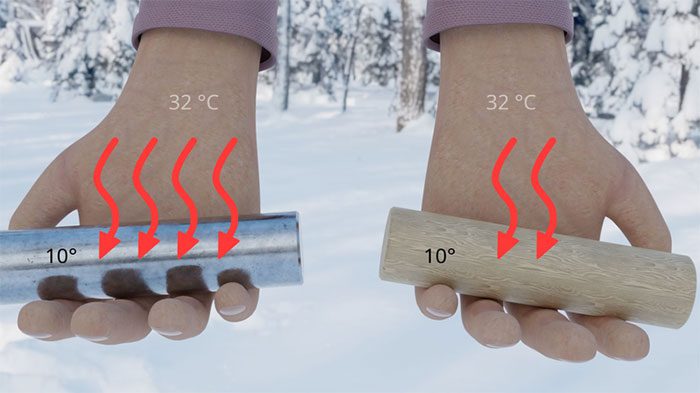
The sensation of cold or hot is actually related to the thermal conductivity of materials.
Derek Muller, a scientist and popular YouTuber on the Veritasium channel, explains: “When you touch something, you are not actually sensing the temperature. You are sensing the rate of thermal energy transfer – from your body outward or vice versa.” In other words, the sensation of cold or hot is indeed related to the thermal conductivity of materials.
Metals are very good conductors of heat, meaning they transfer or absorb heat much faster than other materials like wood or plastic. When you touch a cold metal surface, thermal energy from your hand quickly transfers to the metal, making you feel cold. In contrast, wood or plastic are poor conductors of heat, retaining warmth in your hand longer, creating a warmer sensation.
A Similar Phenomenon with Hot Objects
Interestingly, this principle also applies when the material is hotter than your body. For instance, when you take a cake out of the oven, the metal baking tray will feel much hotter compared to the cake itself. This happens because metal conducts heat very quickly, transferring thermal energy to your hand at a higher rate than the cake does. This is also why you need oven mitts when handling hot metal objects but can hold the cake with bare hands.
Derek Muller clearly illustrated this in an experiment: he placed an ice cube on two surfaces, one aluminum and the other plastic. The results were surprising: the ice cube on the aluminum melted much faster than on the plastic, even though aluminum “feels” colder. This is because aluminum, with its good thermal conductivity, transfers heat to the ice cube more quickly, causing it to melt.
Why Does Metal Conduct Heat Better?
The thermal conductivity of a material depends on how thermal energy moves through it. When a material absorbs heat, the atoms within it begin to vibrate, transferring this energy to neighboring atoms. This process is similar to ripples spreading on the water’s surface when you throw a stone into it.
Metals have a special advantage over wood or plastic: free electrons. In metals, some electrons are not “trapped” by their atoms and can move freely within the metallic structure. When these electrons absorb heat, they move faster and transfer thermal energy more efficiently. This makes metals extremely effective conductors of heat compared to other materials like wood, which has a more complex and less uniform molecular structure that hinders heat transfer.
In Conclusion
So why does metal feel colder than wood, even at the same temperature? Simply because you are not actually sensing the temperature. Instead, you are sensing the rate of thermal energy movement. Metals, thanks to their unique molecular structure and free electrons, are exceptional thermal conductors, which is why they feel colder to the touch – or hotter when they are warmer than your body.


















































