As we know, radioactive elements can cause abnormalities in our bodies. However, enriched uranium at the nuclear level is an exception.

The metal ingot in the image is an alloy of uranium, zirconium, molybdenum, and other metals.
Uranium is known as a radioactive substance and can be used as nuclear fuel. In the impression of many people, to come into contact with this nuclear radioactive material, one must wear protective clothing and be equipped with advanced devices to avoid injury from radiation.

However, for experts knowledgeable about this metal, they can hold it directly in their hands without gloves or protective equipment, but they will need to wash their hands thoroughly afterward.
Unlike chemical bombs, the explosive energy of a nuclear bomb comes from two types of nuclear reactions: nuclear fusion and nuclear fission (the difference between nuclear reactions and chemical reactions is that nuclear reactions occur at the atomic level and can change the types of elements). Taking the atomic bomb as an example, the nuclear material contained in the bomb’s body is uranium enriched to over 90% purity, capable of releasing enormous energy through the fission chain of uranium.

The power of nuclear weapons is extremely terrifying; after the explosion, they can emit extremely strong nuclear radiation, which is released following the fission of a large number of uranium nuclei.
When it comes to radiation, many people feel scared, but understanding radiation correctly will give you a new perspective that changes this perception.
Radiation is the phenomenon of energy diffusing into the outer world through electromagnetic waves or particles, which can be divided into ionizing radiation and non-ionizing radiation. Typically, only ionizing radiation can cause greater harm in a short amount of time. However, non-ionizing radiation is different. Sunlight is electromagnetic radiation emitted by the Sun. Any object with a temperature above absolute zero (-273.15°C) has the ability to emit infrared rays into the outer world; this type of electromagnetic radiation is everywhere and is fundamentally harmless to humans.

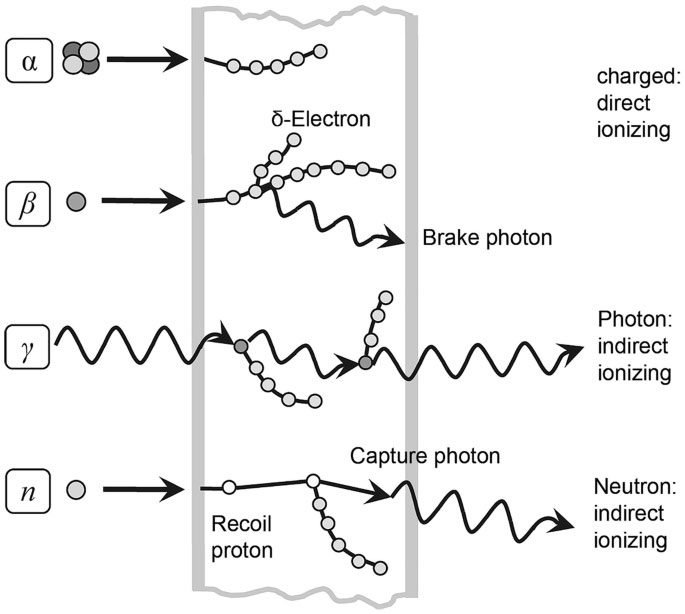
Meanwhile, microscopic particles that exhibit wave-particle duality, ionizing radiation has wavelengths less than 100 nanometers, and nuclear radiation falls under ionizing radiation.
In general, nuclear radiation is released during nuclear fusion or nuclear fission, and nuclear radiation is also released during the decay of radioactive elements (decay is the phenomenon where an unstable nuclear element transforms into a stable nuclear element by releasing rays or particles).
There are many types of nuclear radiation, including alpha rays (helium nuclei), beta rays (electrons), gamma rays (photons), neutrons, positrons, neutrinos, etc. Among them, alpha rays can be blocked by a thin sheet of paper and have very weak penetrating power; beta rays can penetrate slightly stronger and can be blocked by aluminum foil; one of the types of rays with the strongest penetrating power is gamma rays and neutron rays, which require thick lead sheets to block them. When an atomic bomb explodes, a large amount of gamma rays and neutron rays are released.
Uranium (U), atomic number 92, means there are 92 protons in its nucleus. It naturally exists with three isotopes (isotopes are elements with the same number of protons in the nucleus but different numbers of neutrons), specifically uranium-238, uranium-235, and uranium-234, with their natural abundances being 99.28%, 0.71%, and 0.006%, respectively, all of which are radioactive. Uranium-238 was discovered in 1789 by Martin Heinrich Klaproth, while uranium-235 was discovered in 1935 by Canadian scientist Dunstar.
Although the yield of uranium-238 is very abundant and can also undergo fission, it cannot spontaneously undergo chain reactions, thus uranium-238 is also referred to as depleted uranium. However, uranium-235 can do so; its natural abundance is very low, and it usually exists alongside uranium-238, hence they need to be separated to refine uranium-235 to over 90% purity before it can be used to manufacture atomic bombs. High-purity uranium-235 can be obtained through gas diffusion, gas centrifugation, etc., but this process is not simple; typically, 200 tons of uranium ore are required to extract 1 kg of uranium-235.
The nuclear material loaded in atomic bombs is uranium-235 of nuclear grade. Uranium-235 is also used in nuclear power plants, but at that time, the purity of uranium is only about 3%, far inferior to that of nuclear-grade enriched uranium.

For natural uranium ore, its radiation level is even lower.
According to the impression of many people, radioactive substances in general pose extremely high danger. But researchers can actually hold nuclear-grade uranium-235 in their bare hands, not because they are not afraid of death, but because it is truly harmless.
Radioactive elements emit nuclear radiation spontaneously, but when the three natural isotopes of uranium mentioned above decay, they only emit alpha rays, which cannot even penetrate human skin. The outer layer of skin on our bodies is a dead layer, similar to a thin armor that this radiation cannot penetrate.
And the half-lives of these three isotopes (the half-life refers to the time required for a certain number of nuclei to decay to half) are extremely long—about 250,000 years.
In summary, direct contact with nuclear-grade uranium in a short duration will not cause harm to the body. For natural uranium ore, its radiation level is even lower. However, it is important to note that while direct contact is harmless, if uranium enters the human body, it can still be harmful, as it is still radioactive and a heavy metal.
Once uranium is brought into a reactor, the radiation level will be very high and dangerous; even with protective clothing, it should not be touched directly and must be handled with robots.


















































